ENZYMES
Subject: Basic Science Applied to Nursing

Overview
Definition
Enzymes are biological catalysts that boost the rate of chemical processes. Enzymes stay unaltered throughout the procedure. Except for ribozyme, all enzymes are proteins in nature. Each enzyme is identified by the suffix 'ase.' Maltas, Protease, and so on. Maltase is the enzyme responsible for converting Maltose to Glucose and Glucose. Protease is an enzyme that breaks down proteins into peptides and amino acids. However, there are several enzymes that do not have the suffix 'ase,' such as pepsin, trypsin, chymotrypsin, and others. These enzymes are essential for protein breakdown into peptides or amino acids.
Mechanism of Enzyme Action
Figure here illustr ates how enzymes decrease the activation energy of a reaction to enhance its rate (velocity or speed). An activation energy is the quantity of energy needed by reacting molecules to undergo the chemical reaction.
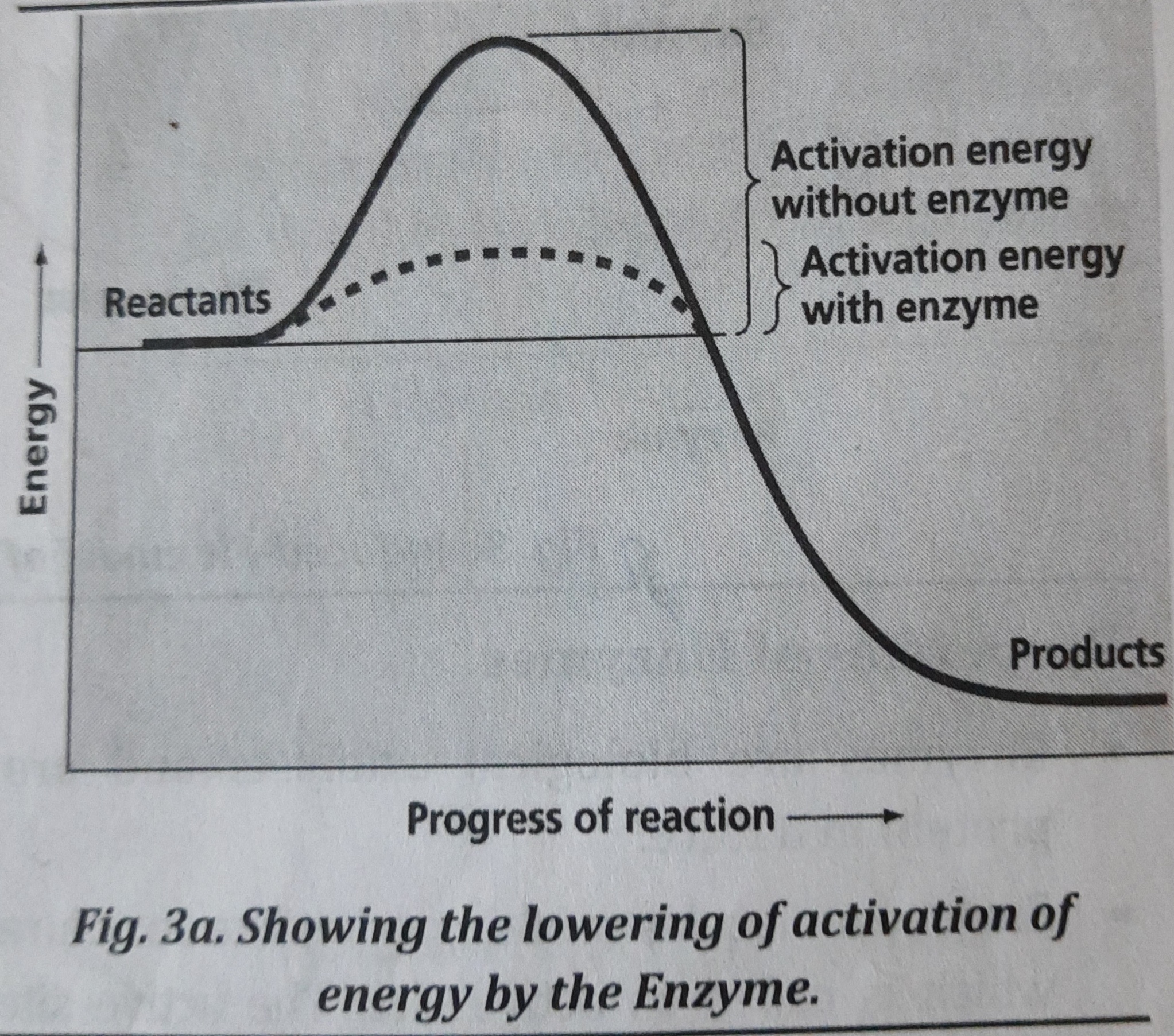
Most cellular reactions occur about a million times faster in the presence of enzyme than they would in the absence of an enzyme. Enzymes react with reactants (called substrate) to produce products.
E + S----ES------E+ P
[here, E = enzyme; s = substrate; ES= enzyme substrate complex; P= Product]
e.g. Lipase reacts with TG to form glycerol and fatty acids.
Enzyme Substrate Binding
Two different theories have been proposed about enzyme substrate binding
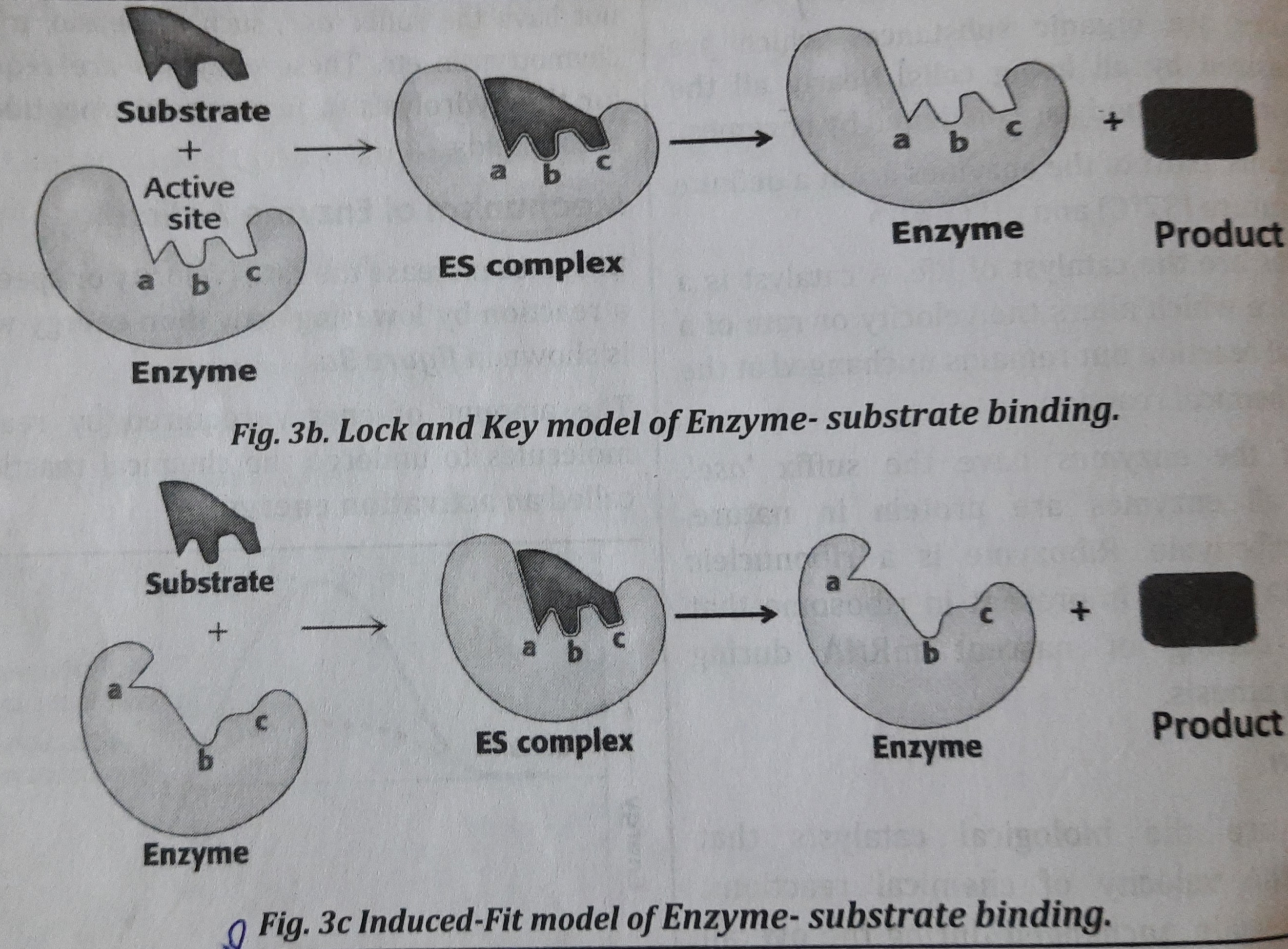
1. Lock-and-Key Model: This model was proposed by Emil Fischer in 1890. In this model, the active site of the unbound enzyme is complementary in shape to that of substrate. Thus, substrate binds on to the enzyme forming enzyme-substrate (ES) complex which ultimately forms product. It is shown in figure.
2. Induced-Fit Model: This model was proposed by Daniel Koshland in 1958. In this model, the enzyme changes shape on substrate binding. The active site forms a shape complementary to the substrate only after the substrate has been bound, forming ES complex which finally forms product. It is shown in figure.
Properties of Enzymes
-
Enzymes are biological catalysts and are protein in nature.
-
Enzyme molecules contain pocket like structure which is called as active site. The active site binds the substrate initially to form ES complex which ultimately form product.
-
Enzyme catalyzed reactions are million times faster than the reactions that would occur in absence of enzyme.
-
Enzymes are highly specific in nature catalyzing only one type of chemical reactions. e.g. Amylase acts polysaccharides, lipase acts on lipids etc. on
-
Regulations- Enzyme activity can be regulated i.e. Enzymes activity can be activated or inhibited. e.g. Coenzyme or Cofactor stimulates the enzyme activity. Accumulations of the products generally decrease the enzyme activity.
Classification of Enzymes
According to IUBMB (International Union of Biochemistry and Molecular Biology), enzymes have been classified into six major classes:
| S.N. | Classes of Enzymes | Examples |
|---|---|---|
| 1. | Oxidoreductases | Acyl CoA dehydrogenase, HMG CoA reductase |
| 2. | Transferase | Hexokinase, Aminotransferase |
| 3. | Hydrolases | All digestive enzymes (amylase, lipase, trypsin) |
| 4. | Lyases | Aldolase, HMG CoA lyase |
| 5. | Isomerases | Triose phosphate isomerase (TIM), Phosohoglucose isomeras |
| 6. | Ligases | Glutamine synthetase, Pyruvate carboxylase |
Factors affecting Enzyme activity
The contact between enzyme and substrate determines enzyme activity. [Note: High enzyme activity means increased speed of the enzymatic reaction which results into increased product formation]. The important factors that influence the speed (velocity) of the enzyme reaction are:
-
Concentration of enzyme
-
Concentration of substrate
-
Effect of temperature
-
Effect of pH
-
Effect of product concentration
-
Effect of activators
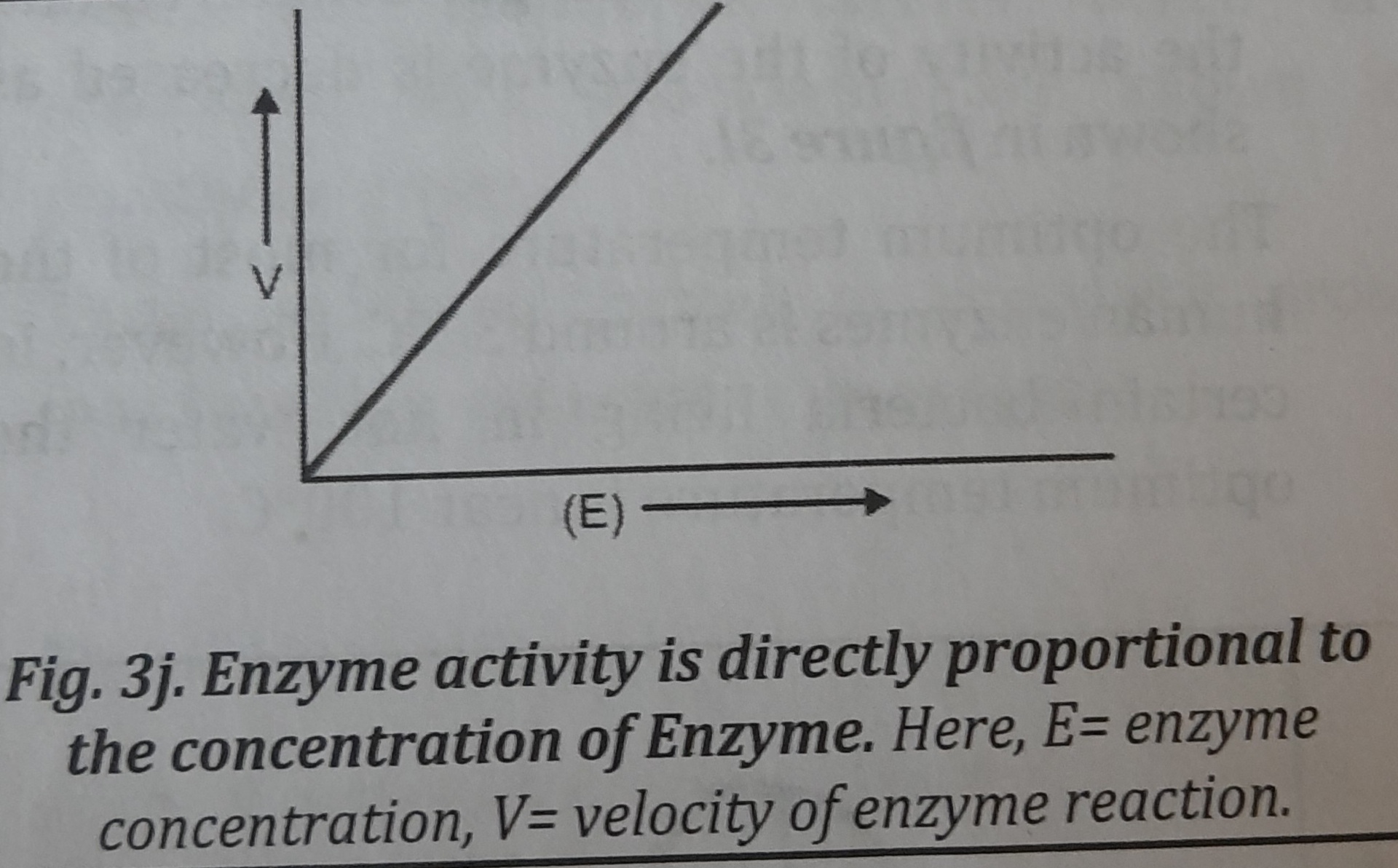
1. Concentration of enzyme: When there is enough substrate present, the rate or velocity (V) of a reaction is directly proportional to the enzyme concentration. As a result, as the concentration of the enzyme increases, so does the reaction's speed and, ultimately, does the concentration of the product. When determining serum enzyme levels to diagnose a disease, this characteristic is used. Figure shows the relationship between reaction speed and enzyme concentration.
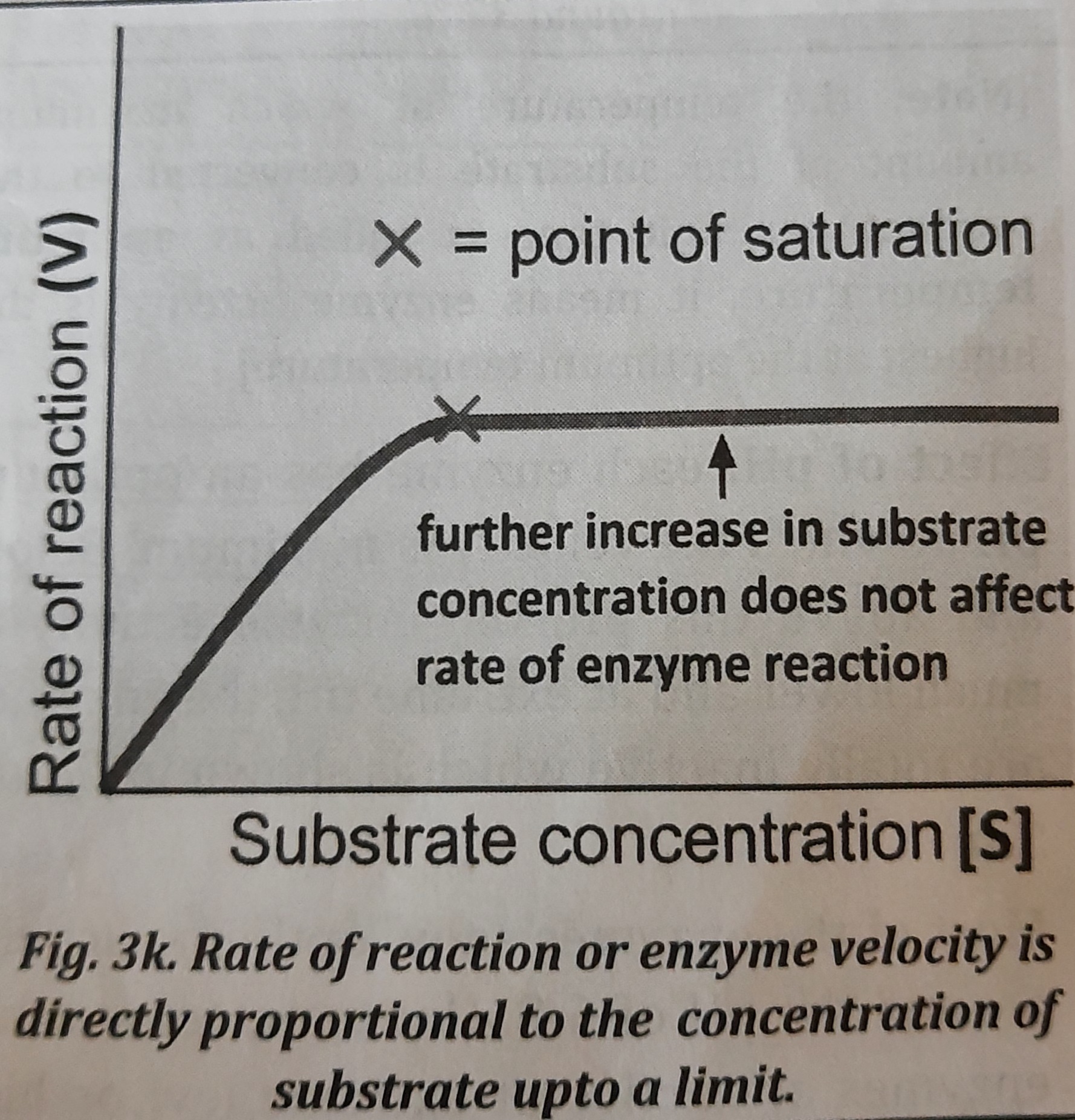
2. Concentration of substrate: When the concentration of substrate is raised, the rate of the enzyme reaction gradually picks up until a certain level of substrate is reached, at which point the rate of the enzyme reaction stabilizes. As a result, within the constrained range of substrate levels, as shown in the figure, as the substrate concentration increases, so does the product concentration.
3. Effect of temperature: The velocity of the enzyme reaction increases as the temperature rises, reaches a maximum, and then decreases. Many enzymes become more active as the temperature rises up to 37°C; however, as the temperature rises over that point, the enzymes become denatured and their activity decreases, as seen in the picture. Most human enzymes function best at a temperature of about 37°C. The ideal temperature for some bacteria, nevertheless, is close to 100°C when they are present in hot water.
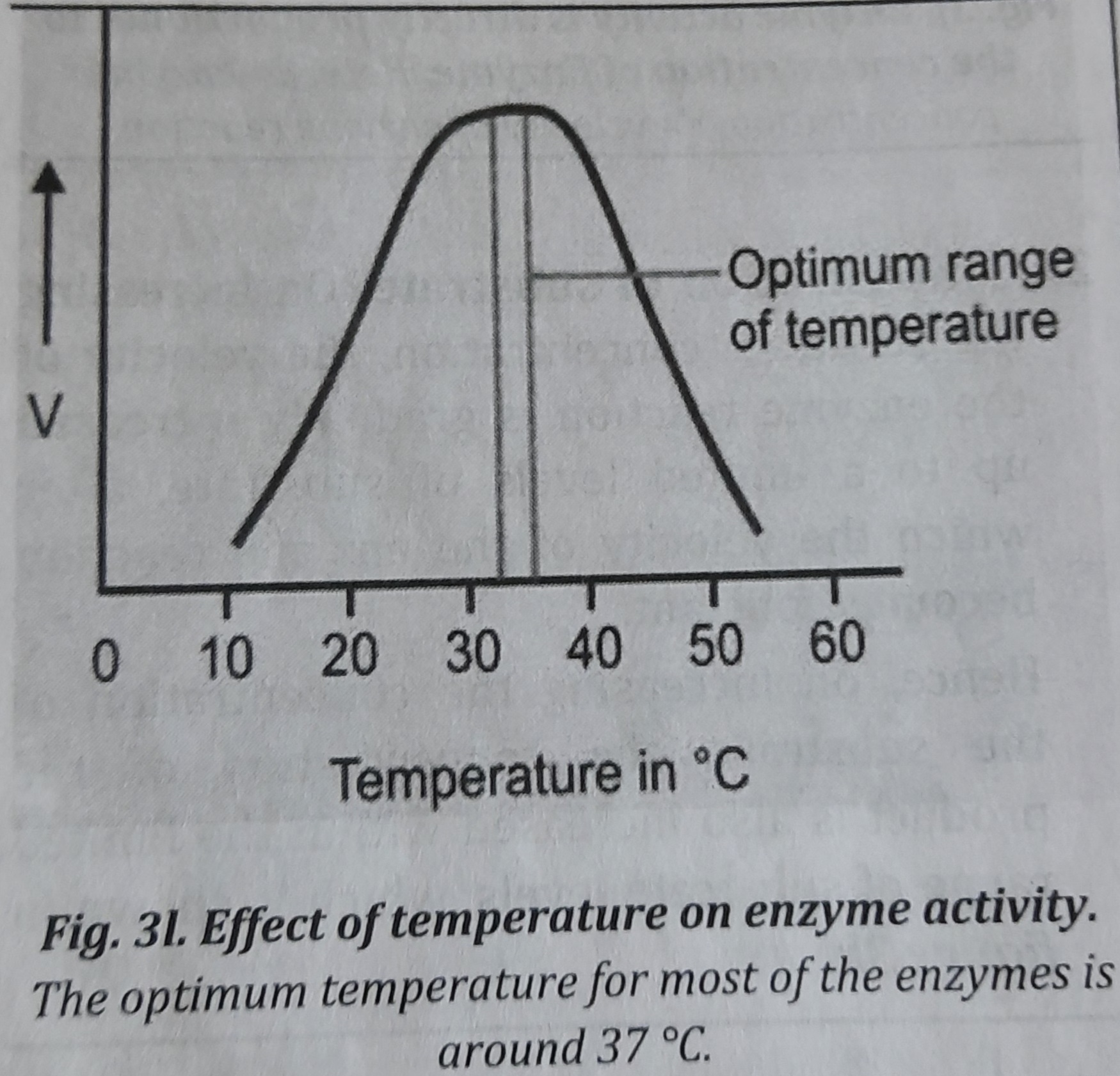
[Note: the temperature at which maximum amount of the substrate is converted to the product per unit time is called as optimum temperature, it means enzyme activity is the highest at the optimum temperature]
4. Effect of pH: Each enzyme has an optimum pH at which the velocity is maximum. Below and above this pH, the enzyme activity is much lower and at extreme pH, the enzymes are totally inactive which is shown in figure. Most of the enzymes show optimum activity around the pH of 6-8. However, some other enzymes are active at extreme low or high pH, such as pepsin (active at pH 1-2), o alkaline phosphatase and arginase (active at (pH 9-10).
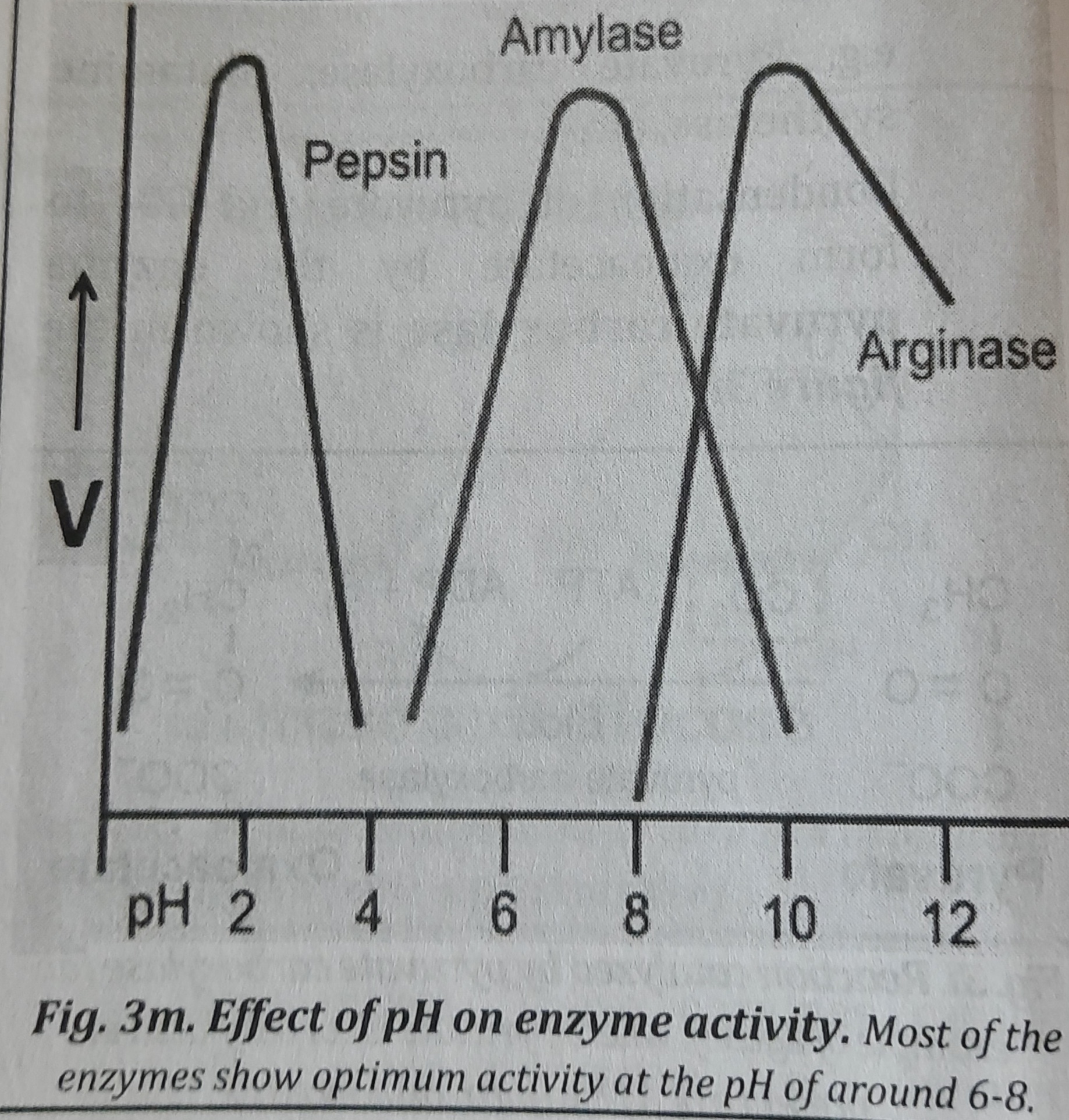
5. Effect of product concentration: The activity of the enzymes is typically inhibited by product accumulation, which reduces the rate of product creation. For instance, bile acid (Cholic acid) buildup decreases the activity of the enzyme 7-a-hydroxylase. This prevents the further conversion of cholesterol to bile acid. Figure illustrates the impact of product concentration on enzyme activity.
6. Effect of activators: Some of the enzymes require additional factors (coenzymes or cofactors) which increase enzyme activity. e.g. Vitamin C (ascorbic acid) is a coenzyme which activates the enzyme 7α-hydroxylase. Once, 7a-hydroxylase is activated, it stimulates the breakdown of cholesterol to form bile acid (cholic acid) and is shown in figure 3n. Similarly, tyrosine hydroxylase requires Copper (Cu); amylase requires Chloride (Cl) for their activity.

Sources of Enzymes
Enzymes are generally synthesized by cells and are released into circulation. Thus, cells are the sources of enzymes. Example: Liver cells are the sources for ALT, cardiac cells are the sources for AST, pancreas and salivary glands are the sources for amylase etc.
Apoenzyme and Holoenzyme
The protein part of enzyme alone is known as apoenzyme, i.e. apoenzyme is the enzyme protein without coenzyme. Apoenzyme may not always be adequate for enzyme activity. So, to become a functional enzyme, the apoenzyme combines with the additional factor to form a holoenzyme. Holoenzyme is complete and can bring about the catalytic activity. Here, the additional factors which are associated with apoenzyme are known as prosthetic group. These additional factors or prosthetic group may be either cofactor or coenzyme.
Cofactors
-
These are the small, inorganic elements and are non-protein in nature. These are required by some enzymes for their activity. e.g. Iron (Fe), Copper (Cu), Magnesium (Mg), Chloride (CI), Potassium (K), etc.
-
The enzymes that require those cofactors (inorganic elements or metal ions) are known as metal activated enzymes or metalloenzymes. e.g. Catalase and peroxidase are iron containing enzymes.
-
Similarly, tyrosinase contains copper, hexokinase requires magnessium, amylase requires chloride, pyruvate kinase requires potassium, etc. Hence, metal ions are required for the activity of respective enzymes.
COENZYMES
Coenzymes are the non-protein, small, additional factors which are derived from organic compounds, mainly from vitamin B complex. Coenzymes expand the catalytic ability of an enzyme in greater amount. i.e. in presence of coenzymes, the activity of enzymes is increased. e.g. Thiamine pyrophosphate (TPP), Pyridoxal phosphate (PLP), Tetrahydrofolate (THF), etc.
Some of the coenzymes derived from vitamin B-complex are tabulated below:
| Examples of Coenzymes | Vitamins |
|---|---|
| Thiamine pyrophosphate (TPP) | Thiamine (B1) |
| Flavin adenine dinucleotide (FAD) | Riboflavin (B2) |
| Nicotinamide adenine dinucleotide (NAD) | Niacin (B3) |
| Pyridoxal phosphate (PLP) | Pyridoxine |
| Tetrahydrofolic acid (THF) | Folic acid |
ISOENZYMES
Isoenzymes are the different forms of the same enzyme which act on the similar types of reactions but are present on different tissue locations. These differ on their structure. Isoenzymes may be the product of the same or different genes.
Example of Isoenzyme:
Isoenzymes of CK: CK1, CK2 and CK3
Isoenzymes of LDH: LDH1, LDH2, LDH3, LDH4, LDH5
Diagnostic importance or Clinical Significance of Enzymes

Things to remember
© 2021 Saralmind. All Rights Reserved.

 Login with google
Login with google