Scope and importance of Bio-chemistry in Health Science
Subject: Basic Science Applied to Nursing

Overview
Definition
The term 'Biochemistry' was introduced by a German Chemist, Carl Neuberg in 1903 AD.
Biochemistry (Greek word, bios= life, chemistry chemical reactions) is the branch of Science which broadly deals with "the chemistry of life and living processes".
The chemical underpinnings of life are a focus of biochemistry. It covers different biomolecules and the chemical processes that occur inside of biological things. Biochemistry examines the various chemical processes that take place inside the living cell, which serves as both the structural and functional unit of all life. So, having a solid understanding of biochemistry is necessary in order to understand anything at the cellular level.
Numerous branches of molecular biology, cell biology, developmental biology, and genetics are included in biochemistry.
Branches of Biochemistry
Biochemistry has following branches
- Medical biochemistry
- Animal biochemistry
- Plant biochemistry
- Cell and Molecular biology
- Microbial Biochemistry
Scope of Biochemistry
Biochemistry is involved in every stage of life, including birth, development, reproduction, aging, and death. Similar to this, there are several biological processes taking place at every second of life.
Because chemical processes in the cell continue to occur wherever there is life, which in turn drives a variety of activities in the organisms, the field of biochemistry is therefore as broad as life itself.
Medical biochemistry's range of application:
In the following professions, biochemistry has application:
Hospital Laboratory: There are openings for medical biochemistry technicians that may do regular examinations to patients who are only visiting the hospital. Clinicians diagnose and treat patients' diseases based on their laboratory test results... Even more patient laboratory test results are crucial to understanding the disease's prognosis.
Research laboratory: Research assistant or research scientist positions may be available for the Biochemistry graduates in hospitals and other research institutions, commercial companies etc.
Teaching Profession: Teaching jobs can also be found at nursing, paramedical and medical schools and universities. A master's degree or PhD are usually eligible for teaching profession.
Pharmaceutical Industries: Biochemistry graduates have role in Pharmaceutical industries for commercial preparations of insulin, progesterone, growth hormone, etc.
Genetic Engineering:Recombinant DNA Technology (RDT) enables the transmission of genes from one individual to another. In this manner, gene therapy can be used to cure hereditary illnesses. One of the first hereditary diseases successfully treated using gene therapy was severe combined immunodeficiency (SCID). Likewise, clotting factor IX, a protein that aids in blood coagulation, is absent in hemophiliacs. Now that a gene for Factor IX has been delivered, patients are experiencing less bleeding episodes.
Importance of Biochemistry
Biochemistry is the study of chemical processes occurring inside living things. For all biological sciences, biochemistry knowledge is a prerequisite. Medical professionals indicate that biochemistry knowledge is necessary for proper illness diagnosis and therapy.
Clinical biochemistry is essential because it enables nurses to frequently check a patient's status, medication dosage, electrolyte balance, and patient education.
Thus, numerous real-world applications of biochemistry assist nurses in becoming proficient and better practitioners.
A knowledge of biochemistry helps understand:
- Hemoglobin's function in the exchange of gases between tissues and the lungs, as well as the chemistry and metabolism of different biomolecules such proteins, lipids, and carbohydrates.
- The removal of waste materials from the body, including urea, creatinine, and uric acid.
- The functions of hormones in living things and the illnesses they are connected with
- Illness detection and appropriate therapy For instance, diabetes is identified based on a high blood sugar level. Similar to this, elevated blood creatinine levels are used to identify renal disease.
- Gene therapy may now be used to treat both the illness and its genetic underpinnings.
- Genetic engineering, also known as recombinant DNA technology, is a biochemical technique that is currently being used to prepare some crucial substances like insulin.
A sound knowledge of Biochemistry deals with the two central concerns of Health Sciences:
• An understanding and maintenance of health: If people are aware of their health, they can maintain their health through dietary control or through proper exercise.
• An understanding and treatment of disease: Understanding the laboratory results help nurses and other health professionals diagnose the disease which in turn help for the effective treatment of the disease.
Solution
Any homogeneous mixture of two or more substances is referred to as a solution. In a solution, one material dissolves into another. One of the two things is always liquid, whereas the other one can either be solid or liquid.
In order to prepare a solution, it is necessary to uniformly dissolve either two liquids, a solid and a liquid, or a gas and a liquid.
In a solution, a solute is typically a solid and a liquid is a solvent.
Solution: Solute (eg, sugar) + Solvent (eg, water)
Different Ways of Expression of Solution:
Concentration is the general term tha expresses the amount of solute present in the given amount of solution. Concentration of solution is defined as the amount of solute present in the given quantity of the solution. Concentration of solution is expressed in different ways:
- Percentage solution
- Molar solution
- Normal solution
Percentage (%) Solution: Solution can be prepared by mixing solute and solvent or solvent and solvent, where final volume is made 100 ml.
Percentage solution can be expressed in different ways:
- Weight by volume (w/v) basis: eg. when 10 gm of sugar is dissolved in water to make a final volume of 100 ml, the solution is called as 10% sugar solution.
- Volume by volume (v/v) basis: eg, when 10 ml of hydrochloric acid (HCI) is mixed with water to make a final volume of 100 ml, the solution is called as 10% HCl solution.
- Weight by weight (w/w) basis: eg, when a solution is prepared by mixing 10 gm of sugar with 90 gm of water, the solution is called as 10% sugar solution.
Molar solution is defined as the number of
gram molecular weight of a substance dissolved in solvent to make a final volume of 1000 millilitre (1 Litre or 1L).
Example:
- Molecular weight of sodium hydroxide (NaOH) is = 40 (23+16+1)
Therefore, if 40 gm of NaOH is dissolved in water to make a final volume of 1 L, it is termed as 1 molar solution of NaOH. i.e. 1 M NaOH contains 40 gm of NaOH in 11 solution.
- Molecular weight of Magnesium hydroxide [Mg(OH)2] Is = 58 (24+32+2).
Therefore, if 58 gm of Mg (OH) is dissolved in water to make a final volume of 1 L, it is termed as 1 molar solution of Mg (OH) 2 ie. 1 M M_{2} (OH) 2 contains 40 gm of Mg (OH) 2 in 1L solution.
Normal Solution: Normal solution is defined as the number of gram equivalent weight of a substance dissolved in solvent to make a solution of 1 liter. Equivalent weight of a substance is calculated by dividing molecular weight with its valency.
Equivalent weight.= Molecular weight/Valency
Example:
Molecular weight of Mg (OH) 2 is = 58 (24 + 32 + 2) .
Equivalent weight of Mg (OH)2 = Molecular weight/ Valency
= 58/2 = 29
[Note: Magnesium hydroxide has two replaceable hydrogen, so its valency is 2. Therefore, equivalent weight of Mg (OH) 2 1 29].
- Hence, if 29 gm of Mg (OH) 2 is mixed with solvent to make a final volume of 1 L, the solution is known as 1 normal solution of Mg (OH) 2 .
- However, if 58gm of Mg (OH) 2 is mixed with solvent to make a final volume of 1L, the solution is known as 1 molar solution of Mg (OH) 2 . [since, molecular weight of Mg (OH) 2 is 58].
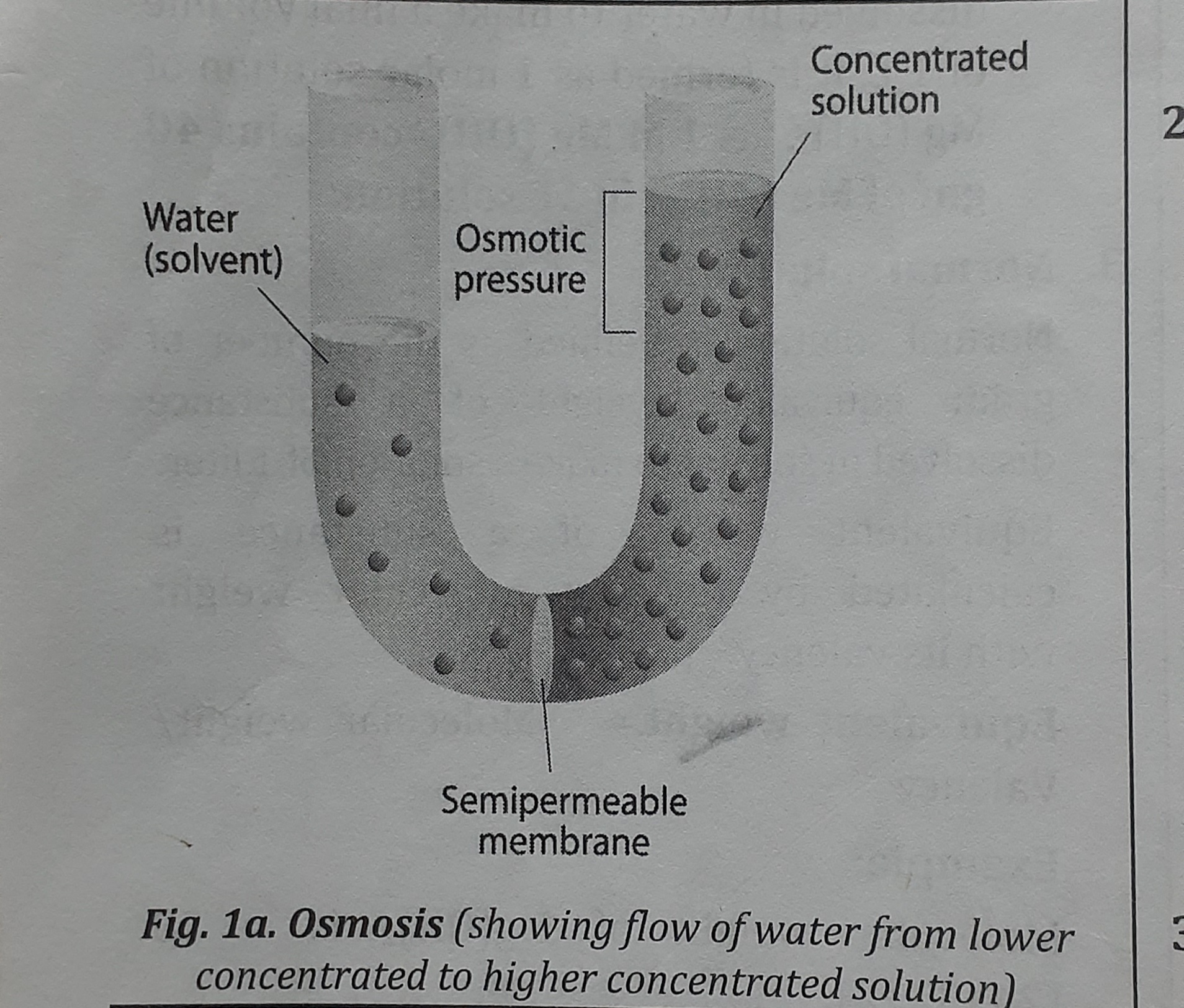
Osmosis
- Osmosis is the term used to describe the movement of a solvent from a solution with a lower concentration to one with a higher concentration (Gk. osmosis = push). From lower to higher, osmosis.
- Solvent passes through a semipermeable membrane.
- Semipermeable membranes are selectively permeable membranes that let solvent but not solute pass through them.
when a cell is placed in the solution having different concentration, the movement of water occurs as follows which is shown in the given figure
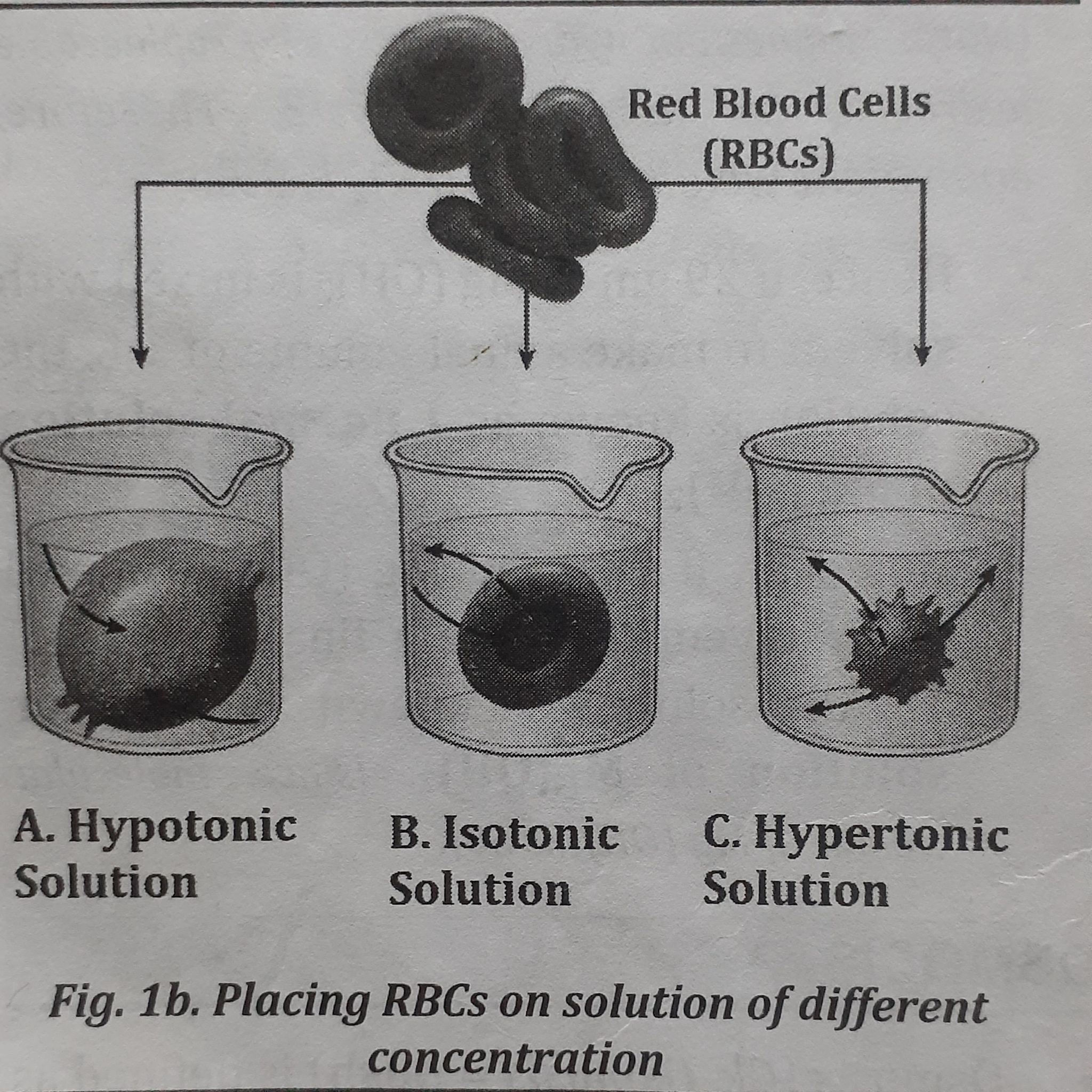
- When a cell (eg. RBC) is placed in a hypotonic Solution (lower concentrated solution):
- Water moves from the solution to inside the cell (because cell has higher concentrated solution) which is shown in figure 1b. A.
- Cell swells and bursts open eg. when raisins are placed in water, the water moves inside the raisins making swell up.
- When a cell is kept in isotonic solution (iso= same or similar):
- Water does not flow in a net direction; it goes equally in both directions.
- The cell size stays constant, as seen in figure 1.b B.
- These solutions are employed in transfusion procedures and wound cleaning. For instance, normal saline (sodium chloride, NaCl) is given to replace fluids lost by the body. Similar to that, wounds are cleaned with isotonic solution.
- When a cell is kept in hypertonic solution (higher concentrated solution):
- Water moves from the cell towards the outer solution (because, the solution outside the cell is more concentrated), which is shown in figure 1b. C.
- Cell shrinks.
[Note: Higher concentrated solution indicates the solution which has more amount of solute, either salt or sugar, etc.]
Applications of Osmosis
- Osmosis is responsible for maintaining fluid equilibrium in the body's intracellular and extracellular compartments.
- Isotonic solution transfusion: For the treatment of dehydration, burns, etc., normal saline (0.9% sodium chloride solution) or dextrose (5% glucose solution) are frequently transfused.
- Washing wounds involves using an isotonic solution. Typical saline, povidone-iodine solution, etc. are examples.
- Laxatives work by preventing the absorption of water from the intestine, which softens the stool and alleviates constipation when taken orally.
Osmotic Pressure
It is defined as the maximum pressure which is applied to a solution to prevent the passage of solvent into it when two solutions separated by SPM.
[For understanding, osmotic pressure is the pressure required to prevent osmosis.]
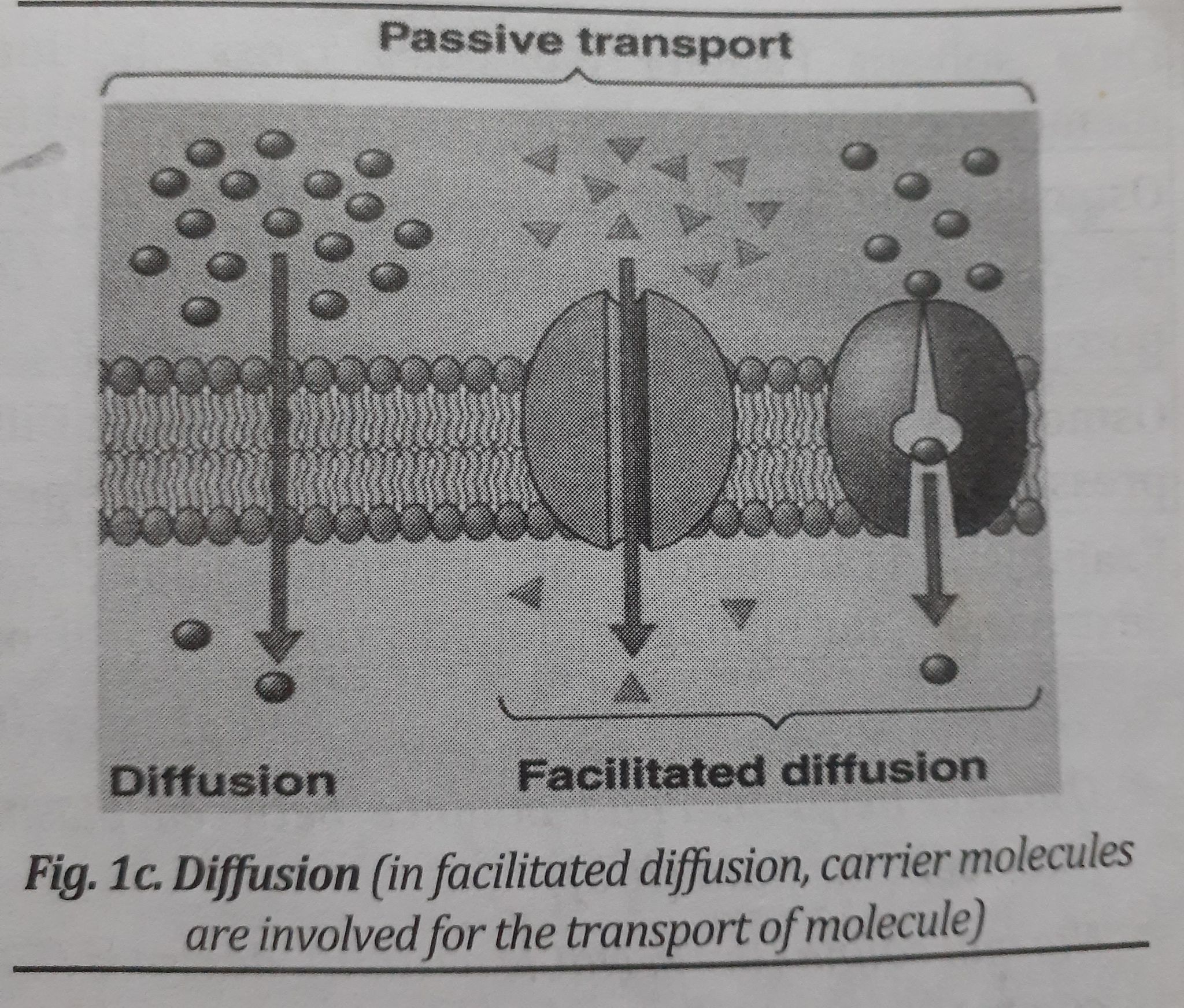
Diffusion
- Diffusion is the movement of molecules from a region of higher concentration to a region of lower concentration (High to Low).
- There is no energy required for diffusion.
- Until all molecules are equally dispersed, diffusion will continue (equilibrium is reached).
- In figure 1.c, the diffusion of molecules is seen.
Types of Diffusion:
Diffusion may be classified as:
- Simple diffusion:
- Simple diffusion occurs from higher to lower concentration.
- Movement or the transport of molecule is a very slow process.
- Facilitated diffusion:
- is a process mediated by the carrier. A protein known as a carrier molecule binds the solute and then releases it inside the cell.
- Molecule transport occurs more quickly than with simple diffusion.
- can move in both directions.
- Molecules with similar structures may compete with one another to prevent entry.
Applications of Diffusion
Exchange of Gases:
Transport of O_{2} from lungs into the blood occurs through diffusion.
Absorption of Nutrients:
Certain nutrients are absorbed by diffusion from the gastrointestinal tract (GIT). eg. water soluble vitamins, pentose sugar, minerals etc.are absorbed through diffusion.
Transport of Nutrients:
Transport of glucose across the cell membrane is an example of facilitated diffusion.
Secretion of Waste Products:
Waste products are eliminated from the body by the process of diffusion.
- eg. Passage of ammonium in renal tubules occurs due to diffusion.
- Carbondioxide (C*O_{2}) is excreted from th cell into the blood stream via diffusion.
In diffusion, particles of the solvent and the solute are both free to travel.
Examples include gas exchange and nutrition absorption from the GI tract.
Neither turgor nor hydrostatic pressure can stop diffusion.
It is independent of the potential of the solute.
Any medium can be used for diffusion.
Differences between Osmosis and Diffusion
| OSMOSIS | DIFFUSION |
| In, osmosis, movement of solvent (water) occurs from lower concentrated solution towards higher concentrated solution through a semipermeable membrane. | In diffusion, movement of molecules occur from a region of higher concentration to a region of lower concentration. |
| Movement of molecules: Lower to higher concentrated solution. | Movement of molecules: Higher to concentration. |
| Semipermeable membrane (SPM) is required. | Semipermeable membrane (SPM) is not required. |
| Only solvent (water) molecules cross the membranes in osmosis. | Both solvent and solute particles are free to move in diffusion. |
| Osmosis can occur only in liquid (water) medium. | Diffusion can operate in any medium. |
| Osmosis is dependent upon the solute potential. | It is not dependent on solute potential. |
| Osmosis is opposed by turgor or hydrostatic pressure of system. | Diffusion is not opposed by turgor or hydrostatic pressure. |
| Example: Transfusion of normal saline, dextrose etc. for managing dehydration. | Example: Exchange of gases, absorption of nutrients from GI tract etc. |
Things to remember
© 2021 Saralmind. All Rights Reserved.

 Login with google
Login with google