PARASITOLOGY
Subject: Basic Science Applied to Nursing

Overview
INTRODUCTION
A relationship between two separate species known as parasitism when one of the organisms (the parasite) benefits from the other (host).
A parasite is a creature that depends on its host for nourishment or shelter. The parasite resides inside or on the host and benefits from it without giving anything back to the host. Furthermore, in the host-parasite interaction, the parasite constantly reaps benefits while the host always suffers consequences.
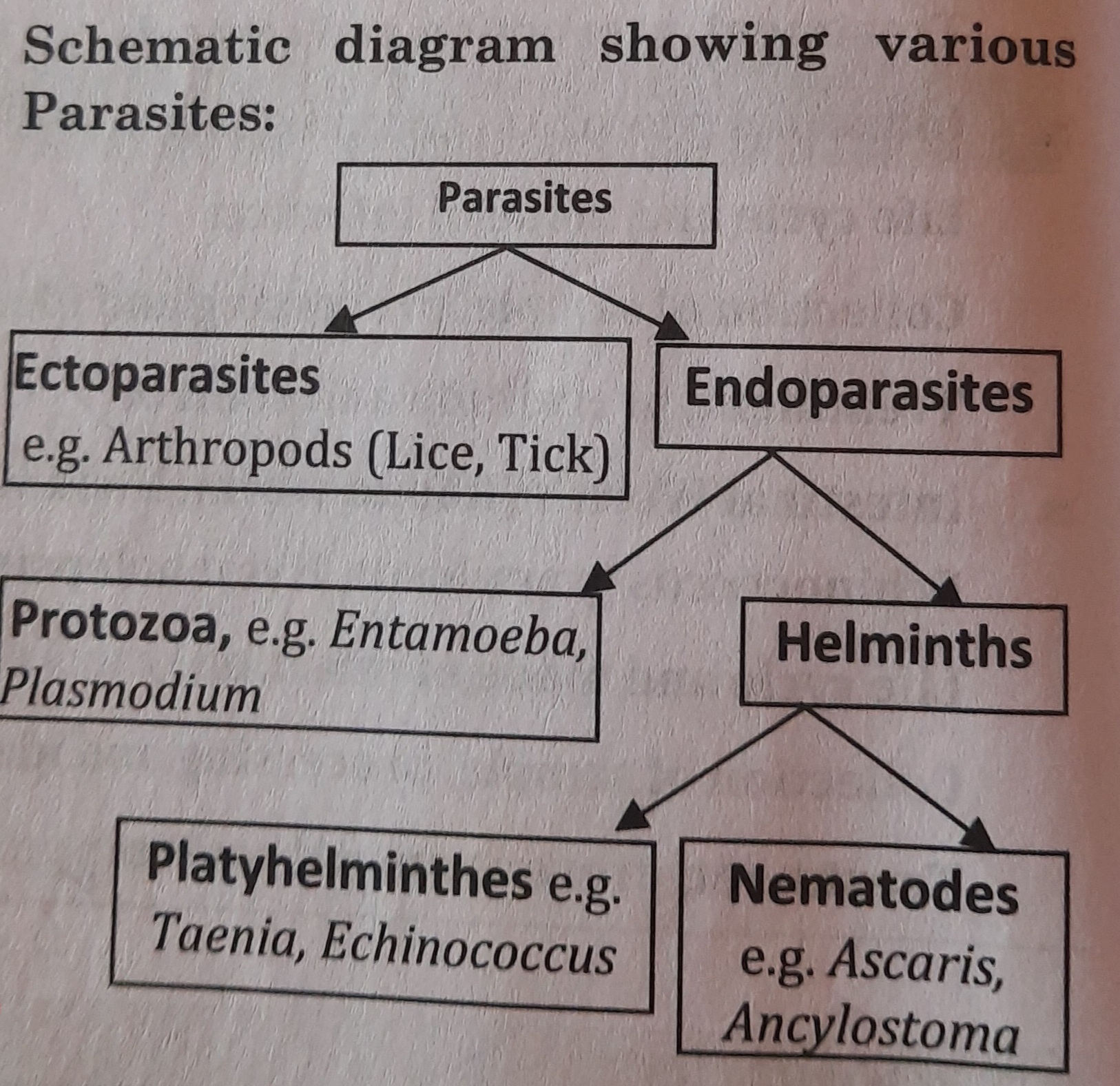
Classification and Characteristics of Human Parasites
-
On the basis of size and cell number, parasites are of two types:
- Microparasites: They are tiny parasites with only one cell. These, such as protozoa, proliferate within their host.
- Macroparasites: These parasites are big and multicellular such as Helminths (Platyhelminthes and Nematoda).
-
On the basis of their location (on host), parasites may be classified into two types-
- Ectoparasites: These are surface-dwelling organisms. such as the louse, tick, and bug.
- Endoparasites: These are housed inside the host's body, helminthic and protozoan human parasites, for instance. Endoparasites can also be divided into the following categories:
- Obligate Parasites: These parasites require a host to survive. Without a proper host, they cannot finish their life cycle. For instance, Toxoplasma gondii, Plasmodium sp., etc.
- Facultative Parasites: Under the right conditions, these parasites can exist as parasites or as free-living organisms. These parasites require a host but can potentially exist without one. Acanthamoeba sp. and Naegleria fowleri, for example.
- Accidental Parasites: These organisms exist as parasites on odd hosts. In humans, for example, Echinococcus granulosus.
- Aberrant or Wandering Parasites: A parasite is considered aberrant if it cannot reach its regular location in the host. The parasite roves and settles in an uncommon organ, where it is unable to finish its life cycle. e.g. In the absence of surgery, tapeworms (Taenia solium) travel to the brain and remain there.
- Free-living: It is the parasite's non-parasitic stage of life in the absence of a host. For instance, in the soil, hookworms (Ancylostoma duodenale) have active, free-living phases.
Routes of Transmission of Parasitic Infestation
- Direct Transmission: Some parasites have the potential to carry illnesses straight from one person to another. The following list includes many means of parasite direct transmission:
- By Sexual Intercourse:
Trichomonas vaginalis, which causes trichomoniasis, is spread through sexual contact. Trichomonas, a protozoan parasite, is passed from one person to another via unprotective sexual contact from the penis to the vagina or from the vagina to the penis. Additionally, it may go from vagina to vagina. Redness of the genitalia and a burning feeling after urination are signs of trichomoniasis. Men may feel discomfort after ejaculating, while women may suffer atypical vaginal bleeding or discharge with an unpleasant odor. - By Kissing:
In the gingival regions around the teeth of humans and other animals, Entamoeba gingivalis can be found. It is frequently spread through direct oral contact and dwells in the gingival tissues of unclean mouths where it is plentiful. via saliva or fomites droplets. Entamoeba gingivalis was first thought to be the cause of pyorrhea, however it is now known that various bacterial infections can induce pyorrhea. - Inhalation:
Eggs of Enterobius vermicularis that are in the air may be ingested. Larvae are created from eggs when they hatch and go to the colon, where the females move to the perianal region and itch the anus. - Congenital Infection:
Toxoplasma gondii and Plasmodium spp. can spread transplacentally from a woman to her fetus.
- By Sexual Intercourse:
- Oral Transmission:
It is the most typical way for parasites to spread. When infected meals, water, or plants are consumed, many intestinal parasites enter the body. Parasitic infection may happen if the infective stages of the parasites, such as cysts, embryonated eggs, or larval forms, are ingested. When infective cysts are ingested, infection with Entamoeba and other intestinal protozoa happens. When embryonated eggs are ingested, infection with roundworms, whipworms, or pinworms results. Similarly, when adult larvae are ingested through raw meat, tapeworm infection results. Therefore, the source of infection that may be spread by oral channel is contaminated food and drink, filthy fingers, and fomites (such as clothes, utensils, which are likely to carry parasites or their infective stages). - Penetration of Skin:
Some parasite larvae may penetrate the skin and enter the body. Infestation with hookworms happens when Ancylostoma duodenale larvae pierce the skin of a person walking barefoot on contaminated soil. In a similar manner, Schistosoma infection happens when waterborne organisms' larvae enter the skin. - Vector Borne Transmission:
Many parasitic diseases, including malaria and filariasis, are spread by blood-sucking arthropods. Other parasitic diseases, like amoebiasis, are spread by houseflies carrying amoebic cysts from feces to food. These arthropods are referred to be vectors because they aid in the spread of illness. There are two sorts of vectors:- Biological Vectors:
Biological vectors are those that enable the formation or proliferation of parasites. For instance, female Anopheles mosquitoes may sexually reproduce Plasmodium. Similar to humans, Culex mosquitoes grow microfilariae into third stage larvae, which are infectious and cause filariasis. The biological vectors in this case are mosquitoes. - Mechanical Vectors:
Mechanical vectors are those that do not allow parasites to reproduce or alter in any way. These are the parasite vectors that just spread infectious parasites. e.g. Houseflies are referred to as mechanical vectors because they act passively to transport amoebic cysts from feces to meals.
- Biological Vectors:
Parasitic Diseases Due to Poor Hygiene:
- Amoebiasis
- Giardiasis
- Hookworm infestation
- Pinworm infection
- Ascariasis
- Scabies infection
- Pediculosis
Blood Parasites (Malaria Parasite, Kalazar, Microfilaria)
Parasites that are transmitted by blood often spend a portion of their life cycle in the blood of their host. These parasites can spread either way:
- through the exchange of needles or by the transmission of contaminated blood during blood transfusions, or,
- with the use of a vector, such as a bitten host by an infected insect that releases the parasites into the circulation.
Each year, blood-borne parasites kill thousands of people in developing and poor nations.
Some common blood borne parasites include-
- Plasmodium- causes malaria
- Leishmania- causes Kalazar
- Trypanosoma- causes sleeping sickness or chaga's disease
- Wuchereria bancrofti- causes filariasis
- Schistosoma- cause endemic hematuria, gastro-intestinal haemorrhage.
Malaria Parasite
- Malaria is brought on by the malaria parasite. Plasmodium is the scientific term for the protozoan parasite that causes malaria.
- Human erythrocytes (RBC) and hepatocytes (liver cells) contain the renowned intracellular parasite Plasmodium.
- Four of the many species of this parasite are known to infect people and cause malaria., they are-
-P. vivax
-P. falciparum
-P. malariae
-P. ovale
- . Of these four species, P. vivax and p falciparum account for 95% of human infections.
Morphology
Structurally, Plasmodium is dimorphic (found in two forms):
- Trophozoite: it is a feeding stage, the shape of the body is not fixed.
- Sporozoite: it is microscopic, spindle or sickle shaped.
Size: length-14 µ (micron) and breadth- 1 μ.
Life cycle
Plasmodium is a digenetic parasite as it completes its life cycle in two different hosts-
- Intermediate or Secondary host: Human, in whom asexual cycle of malaria parasite is completed.
- Definitve or Primary host: Female Anopheles mosquito, in which sexual reproduction of Plasmodium takes place.
Life Cycle in Human (Schizogony/ Asexual Cycle)
Sporozoites, which are infectious types of parasites, are found in the salivary glands of infected female Anopheles mosquitoes.
Sporozoites are introduced into a man's blood stream via a mosquito bite.
The cycle of malarial parasites in man comprises following stages:
- Pre-erythrocytic schizogony
- Erythrocytic schizogony
- Gametogony
- Secondary exoerythrocytic schizogony
Pre-erythrocytic Cycle:
A lot of sporozoites are injected into the blood of a healthy man by an infected female anopheles mosquito, where they then enter the hepatic cells. Multiple divisions occur inside sporozoites as they transition into schizont. Merozoites are discharged into the bloodstream as a result of liver cell rupture.
Erythrocytic Cycle
- The merozoites now penetrate and proliferate inside the red blood cells (RBCs). They go through the trophozoite, schizont, and merozoite phases here. Merozoites are released when RBCs rupture, and they infect newly formed RBCs. Malaria attacks manifest clinically during this cycle of parasitic multiplication.
- Cryptozoite, the stage of the trophozoite that feeds, consumes the cytoplasm of RBCS. Trophozoite grows a vacuole and pushes the nucleus to one end of the cell, a process known as the "signet ring stage" (signet ring-like). Later, the trophozoite splits into merozoites with haemozoin granules by numerous fissions.
- When the RBC wall reaches its maximum size, it bursts, releasing a significant amount of merozoites into the bloodstream.
Gametogony
Following erythrocytic schizogony, some merozoites transform inside red blood cells into gametocytes known as microgametocytes and macrogametocytes, respectively, which are male and female. Peripheral blood of hosts only contains developed gametocytes, and these hosts (humans) are referred to be carriers.
Secondary Exoerythrocytic Schizogony
Some sporozoites from P. vivax and P. ovale reside in the latent (resting) stage in liver cells. Hypnozoite refers to the parasite's dormant stage. A relapse of malaria results from hypnozoites being reactivated, developing, and releasing merozoites after a period of weeks, months, or years (typically up to two years).
Life Cycle in Female Anopheles Mosquito (Sexual Cycle/Sporogony)
- Female Anopheles mosquitoes puncture human skin as part of their blood diet and consume the parasites (gametocytes). Considering that female Anopheles have a powerful proboscis that is able to puncture flesh.
- Only the fully developed sexual forms of the parasite may survive within the mosquito; the rest perish.
- Fertilization takes place to create a zygote in the mosquito's midgut. The zygote transforms into an ookinete and ultimately an oocyst. Numerous sporozoites (between 100 and 1000) form inside each oocyst.
- Numerous sporozoites are released upon oocyst rupture and are then spread across different mosquito species. body parts
- The mosquito can now spread the illness to new human hosts. Figure depicts the malaria parasite life cycle.
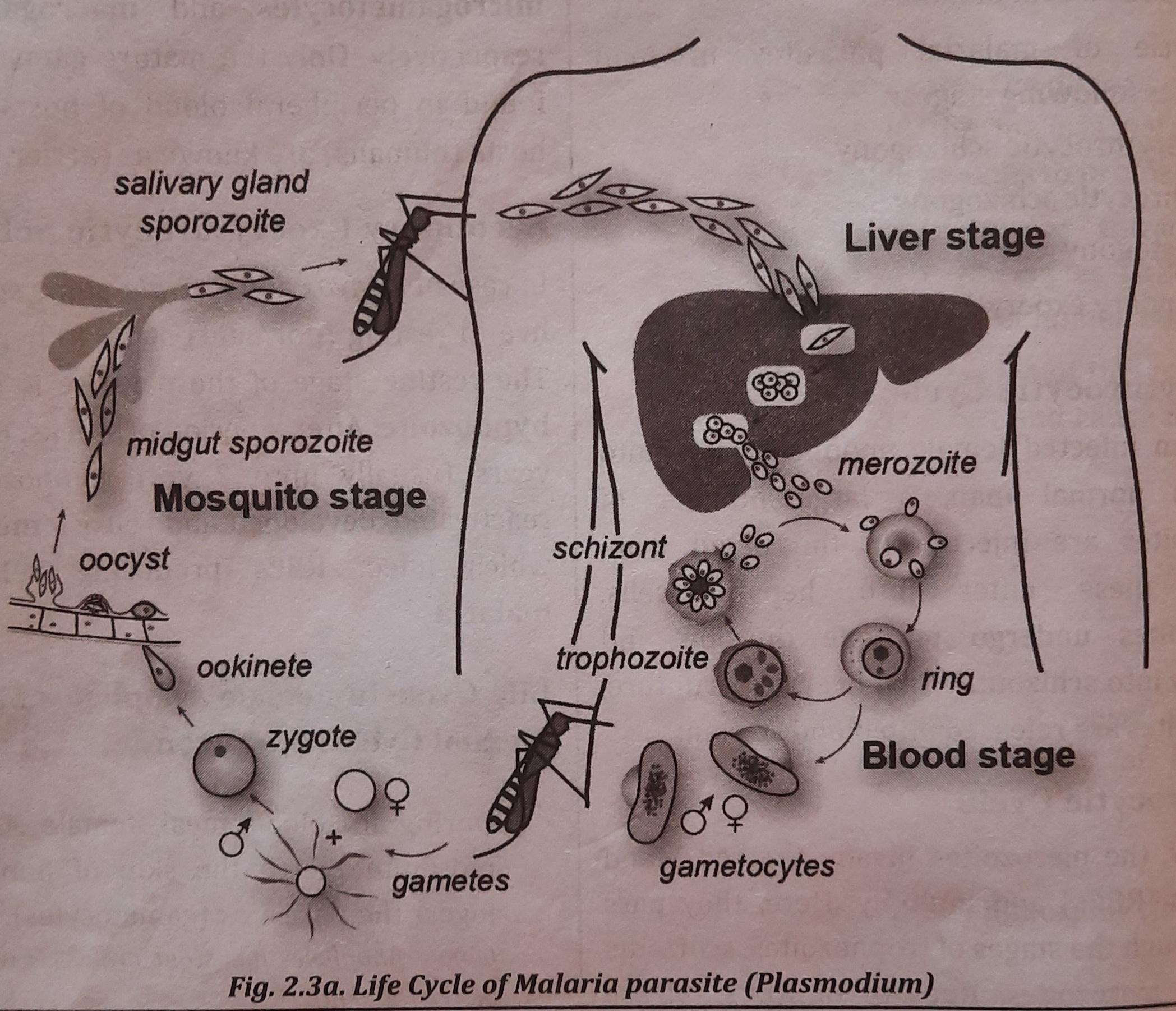
Infective Stage
Infective stage of Plasmodium (malaria parasite) is sporozoite.
Mode of Infection (Method of Transmission)
- Vector Transmission: Infected female Anopheles mosquitoes transmit the sporozoites when it pierces the skin of man.
- Other Mode of Transmission: By injecting blood of malaria patient to other
- by blood transfusion: blood transfusion from infected donor to healthy person.
- congenital malaria: congenital infection of new born from an infected mother.
- by the use of contaminated syringes particularly in drug addicts.
Incubation Period: Incubation period varies for various Plasmodium.
For P. falciparum, it is 10-14 days; for P. vivax and P. ovale, it is 13-17 days, and for P malariae, it is 28-30 days.
Clinical Findings
- Fever and chills, anemia, and splenomegaly are the main clinical manifestations of malaria.
- Headache, myalgias (muscle pain), and arthralgias are other symptoms (pain in joint).
- Other symptoms include stomach discomfort, nausea, and vomiting.
- P. falciparum malaria, which is lethal if left untreated, damages the kidneys and the brain and results in cerebral malaria (blackwater fever).
Collection of Sample, Processing and Identification
Sample: Blood sample is collected for the diagnosis of the disease.
- The condition can be identified by microscopic examination of the parasite in the blood smear.
- Malaria may be diagnosed by looking at a signet ring (Trophozoite).
- An individual patient's blood sample is used in the quick diagnostic test to find malaria antigens.
Prevention and Control
Preventive measures are of two types:
- Primary Preventive Measures:
These are the prevention from the infection, such as- usage of anti-mosquito lotion and mosquito netting.
- by micronetting the vents, windows, and doors.
- maintaining good health and boosting the body's defense mechanisms.
- Secondary Preventive Measures:
These include various methods of destruction or repulsion of the Anopheles mosquitoes. Secondary measures include-- manual mosquito eradication (killing of mosquito by hand).
- pesticides like DDT and pyrethrum are sprayed, among others.
Treatment
- Chloroquine was once the go-to medication for treating malaria, but P. falciparum has developed a resistance to it.
- The most effective medication for treating malaria is quinine.
- Both tetracycline and clindamycin are anti-malarial.
Kalazar Parasite
The intestinal parasite Kalazar, also known as Leishmania, is a member of the phylum Protozoa. The disease known as leishmaniasis is caused by a variety of species in the genus Leishmania.
The various species of Leishmania are-
- L. donovani
- L. infantum
- L. tropica
- L. major etc.
Leishmania donovani
- It is an obligatory intracellular parasite of human and other vertebrate reticuloendothelial cells (liver, spleen, bone marrow). The most dangerous kind of the illness, visceral lesimaniasis, often known as dum dum fever or kala-azar, is caused by L. donovani.
- Leishmaniasis is a group of diseases, each with its own clinical manifestations and epidemiology, that are spread to humans by the bite of female sandflies (Phlebotomus).
Morphology
The parasite exists in two morphological forms-
- Amastigote form: These are spherical or oval-shaped, non-motile, and range in length from 2-4 um. These can be found in human reticuloendothelial cells (vertebrate host).
- Promasigote form: These are elongated, motile structures that range in size from 1.5 to 3.5 m in width and 15 to 25 m in length. These are located in the sandfly's digestive system (insect vector).
Life cycle
Leishmania donovani is a digenetic parasite-
Definitive host-man and other vertebrates.
Intermediate host- female sandfly (Phlebotomus), a vector.
- The parasite's amastigote form is present in the bloodstream of infected people.
- The human amastigotes that enter the sandfly's midgut during a blood meal are consumed by the sandfly. Amastigotes change into promastigotes and proliferate in astronomical numbers.
- Promastigotes go to the buccal cavity and throat. The promastigotes are deposited/released in the pierced wound when a sandfly stings the victim.
- The promastigotes go inside human reticulo-endothelial cells. These are multiplied through binary fission and are encircled by macrophages.
- Macrophage rupture releases amastigotes.
- some of which may be phagocytosed by other macrophages.
- amastigotes in peripheral blood and skin of human are ingested by sandflies during blood meal, thus, repeat the cycle. Its life cycle is depicted in figure.
_1668223826.jpg)
- The sandfly consumes amastigotes.
- Amastigotes divide by binary fission to create promastigotes.
- Pharynx with mastigotes.
- One gets bit by a sandfly.
- The puncture wound becomes a deposit location for promastigotes.
- macrophage phagocytosis, resulting in the formation of amastigotes.
- Amastigotes are multiplied.
- The amastigotes are released when the macrophage bursts.
- Other macrophages phagocytose some amastigotes.
- Sandflies feed on amastigotes found in peripheral blood and skin, continuing the cycle.
Infective Stage
Promastigotes (promastigote forms) are the infective stage.
Mode of Infection
Kalazar is transmitted when an infected female sandfly (Phlebotomus) transmits the promastigotes to human host.
Pathogenesis
L. donovani causes visceral leishmaniasis or Dum Dum fever or tropical kala-azar, splenomegaly.
Symptoms: Fever, splenomegaly, lymphadenopathy, black sickness (kala= black; azar= sickness) etc.
Collection of Sample, Processing and Identification
The sample is taken in an aseptic environment. Blood, bone marrow, and spleen aspirate or biopsy are all included in the sample.
- Microscopic Demonstration of Parasite:
- Microscopically seeing the amastigote develop inside monocytes in peripheral blood
- The most typical diagnostic sample obtained is a bone marrow aspirate or biopsy. aspiration from tissues such the lymph nodes, liver, or spleen. The smears stained with Giemsa stain show amastigote formations.
- The most accurate specimen for showing parasites is a spleen aspirate.
- Immunological Tests:
- Aldehyde test: a drop of 40% formalin is added to 1 ml of serum, coagulation of serum indicates positive test.
- ELISA (Enzyme linked immunosorbant assay) using species specific monoclonal antibodies are used.
- Non-specific Laboratory Test:
- Blood Count: pancytopenia, mainly neutropenia and decreased erythrocyte count.
- Hemoglobin Estimation: decreased Hb reveals anemia.
Prevention and Control
Leishmaniasis may be prevented and controlled by treating all cases, eliminating the vector (sandfly), and practicing personal prophylaxis by taking anti-sandfly precautions.
Following measures can be implemented for prevention and control of leishmaniasis
- Control of sandflies by spraying the insecticides such as DDT (Dichlorodiphenyltrichloroethane).
- Remove unnecessary vegetation surrounding houses, which my harbor or breed sandflies.
- Periodic fumigation and mosquito net can be used to be safe from sandfly attack.
- Treatment of vertebrate host (such as infected human or dog etc.) as early as the diagnosis is made.
Treatment
- Sodium stibogluconate: 600 mg daily for 6 days, IV.
- Aminosidine (paromomycin): 14 mg/kg body weight daily, IM.
- Pentamidine: 4mg / k * g / day for 10 days, IM.
- Amphotericin: 0.25 to 1 mg/kg/day, as slow infusion for upto 8 weeks.
- Allopurinol: has also been reported to be effective.
Microfilaria
The larval form of Wuchereria bancrofti, also known as Filaria bancrofti, was first detected in the hydrocele fluid of a Cuban patient. The nocturnal periodicity is visible in microfilaria.
circulates in peripheral blood at night
vanish from circulation during the day and accumulate in the lungs.
Man's lymphatic vessels and lymph nodes are home to adult worms.
Morphology
Adult worms: long, transparent, hair like.
Size Male: 2.4-4 cm long and 0.1 mm thick.
Female: 8-10 cm long and 0.2-0.3 mm thick. The female is viviparous and liberates microfilariae into lymph from where they reach to blood.
Life Cycle
Wuchereria is a digenetic parasite i.e. it completes its life cycle in two hosts- human and mosquito.
Definitive host: Human
Intermediate host: Female mosquitoes (Culex, Aedes and Anopheles).
Stages in Man
- Adult worms reside in lymph nodes and. lymphatics (usually scrotal and abdominal etc.) of man. Male fertilizes female and the gravid female gives birth to microfilariae.
- Now, the microfilariae reach into general circultion, appears in blood 2 hours before and after midnight and for the rest of the period, these remain in lungs (pulmonary circulation).
Stages in Mosquito
- Microfilariae are sucked by the mosquito from the blood of human.
- In the stomach of mosquito, the sheath of microfilariae is digested, the larvae then migrate to thoracic muscles.
- In couple of days, microfilariae a metamorphose into 'the first stage larvae'. The first stage larvae moult once or twice to become 'second stage larvae' which is developed to form 'the third stage larvae'.
- The 'third stage larvae' migrate into the labium/proboscis of mosquito as an infective stage.
- When the mosquito bites a normal human, infective larvae (filariform or third stage larvae) enter into the human lymphatic system where they develop into adult worms and obstruct the lymphatic system.
- Male worm fertilizes female worm, gravid female gives birth to microfilariae and the cycle is repeated. The life cycle of Wuchereria is shown in figure
_1668228484.jpg)
Infective Stage: third stage larvae (filariform larvae)
Mode of Infection: When infected female mosquito bites to human hosts, the infective stage larva is transferred to human, where they develop into adult and show the symptoms of the disease.
Pathogenesis
Infection caused by W. bancrofti is known as wuchereriasis or bancroftian fialriasis.
Symptoms:
Filarial fever and headache, enlarged lymph nodes. In heavy infection, the adult worms block lymphatic vessels and glands resulting into elephantiasis.
Elephantiasis of limbs, breasts or vulva in females and elephantiasis of limbs, scrotum, penis, hydrocoele, orchitis and epididymitis are common along with chyluria in males.
Collection of Sample, Processing and Identification
Blood sample is collected for the diagnosis of filariasis. The blood sample must be collected during midnight (10 pm -2 am). In adult, blood is collected from the ear or finger and in infants from the heels.
- Microfilariae are checked for on a blood smear using a microscope. Giemsa or hematoxylin are used to stain smears.
- Additionally, microfilariae may be seen in the hydrocoele fluid and chylous urine.
Prevention and Control
- Bancroftian filariasis can be prevented by control of vectors (mosquitoes) by
- pesticides, such as DDT and malathion, are sprayed.
- introducing environmentally friendly fish to the pond, which reduces mosquito population..using mosquito nets or repellents when sleeping at night.
- prevention of mosquito reproduction
- Bancroftian filariasis can be controlled by treating human hosts on early diagnosis.
Treatment
The drug of choice for treatment of bancroftian filariasis are
- Diethylcarbamazine (DEC), 6mg/kg body weight, daily for 12 days.
- Albendazole and Diethylcarbamazine are effective in combined form.
Intestinal Protozoa
Entamoeba histolytica
It is a intestinal parasite residing in large intestine (colon) of man. It belongs to phylum protozoa. Entamoeba histolytica (histos= tissue, lysis= dissolve) is commonly called as tissue dissolving parasite which cause amoebiasis.
Morphology
The parasite exists in three morphological forms, which is shown in figure 2.4a.
- Trophozoite
It is the feeding, growing or adult stage. It is comparatively larger form measuring about 20-30 µm in diameter.
- Precyst
It is oval with a blunt pseudopodium and smaller in size measuring 10-20 μm in diameter.
- Cyst
It is spherical, 10-20 µm in diameter. It is surrounded by a thick chitinous wall which makes it highly resistant to the gastric acid, adverse environmental conditions and chlorine found in potable water.
Life Cycle
It passes its life cycle in only one host, i.e. it is monogenetic parasite.
- An entamoeba living in the colon excretes trophozoites in diarrheal stools and cysts in feces. Even if they are consumed by a new host, trophozoites in diarrheal stools cannot live outside the body or in the stomach environment.
- Man gets sick when he consumes developed cysts from contaminated food, drink, or hands. Anal-oral sexual activities can also spread an infection.
- Trypsin, a protein-digesting enzyme, ruptures the cyst wall in the colon, releasing the metacyst (tetranucleate amoeba). Eight new nuclei are created from the binary fission of each nucleus, creating eight miniature amoebae (Metacystic trophozoite).
- Metacystic trophozoites enter the large intestine, spread throughout the tissues, and ultimately settle in the submucous tissue. Binary fission is used to develop and proliferate in this area.
- The trophozoites undergo precyst and then quadrinucleate cyst maturation in the large intestine lumen (infective form of parasite).
- A proteolytic enzyme that E. histolytica secretes during growth causes the necrosis and destruction of tissues in the large intestine. Amoebic dysentery, at this stage, is characterized by the excretion of high numbers of trophozoites, coupled with blood and mucus, in stools. Figure depicts the E. histolytica life cycle.
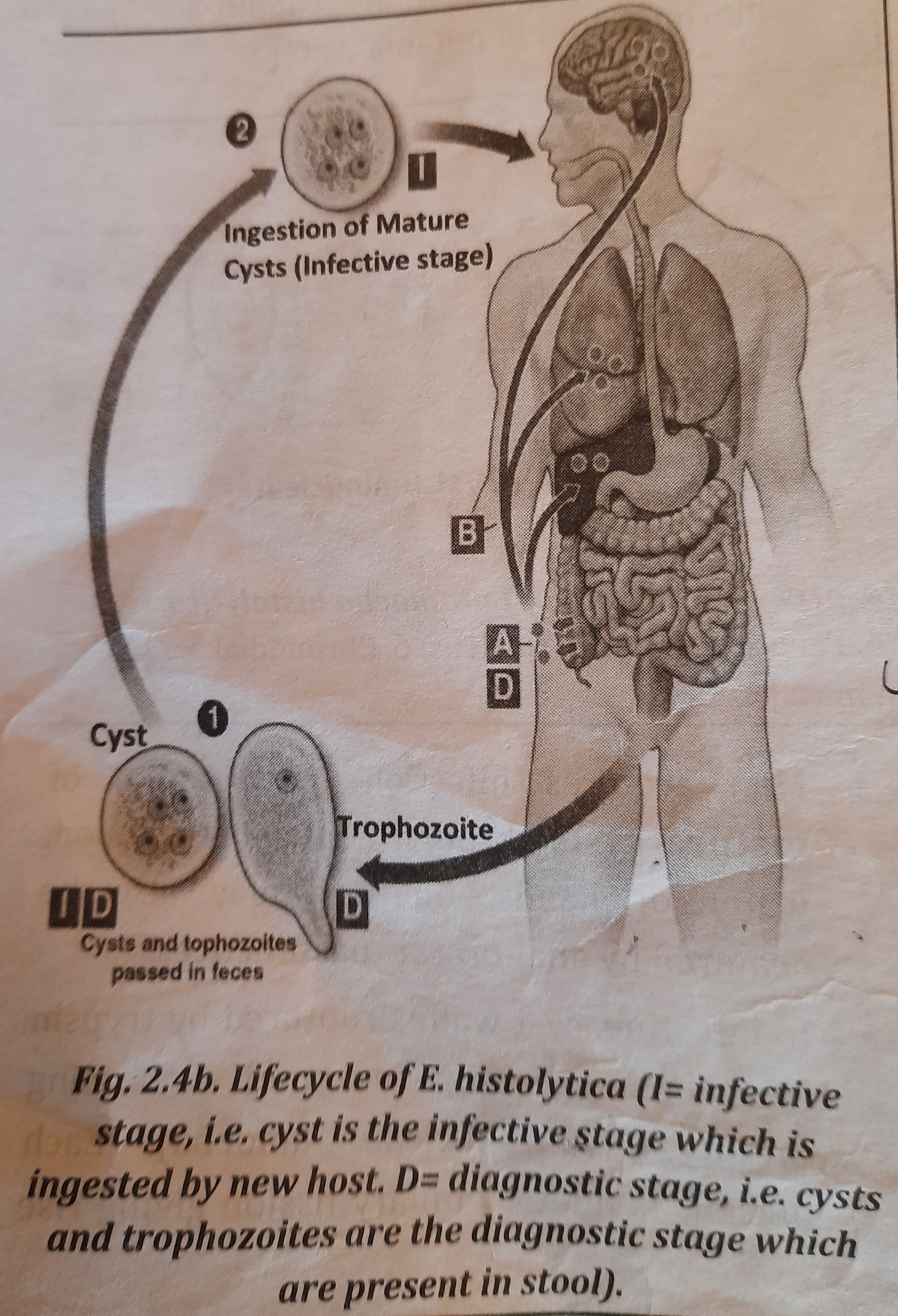
Infective Stage
Quadrinucleate cysts are the infective stage.
Mode of Infection
Quadrinucleate cysts allow E. histolytica to spread from person to person. When a guy is infected, his feces contain quadrinucleate cysts that are released. The host becomes infected when a different host (another healthy male) consumes quadrinucleate cyst through tainted food or drink.
Cysts are carried by houseflies to food and drink, where they are ultimately inhaled by people through feces.
Pathogenesis
E. histolytica causes amoebiasis (amoebic dysentery or amoebic liver abscess). The parasite secretes tissue dissolving enzymes that spoil the epithelial lining of colon. When the parasite reaches the tissues, may cause tissue destruction.
Symptoms
- severe stomach discomfort, diarrhea, and mucus with blood.
- severe disease-stage bleeding ulcers that resemble flasks.
- severe pain in the organs where the parasite has caused damage.
- Patients experience fever, nauseousness, headaches, and fatigue.
Collection of Sample, Processing and Identification
Stool is collected and transported to laboratory immediately.
- Microscopic Observation
Observation of trophozoites of E. histolytica under Microscope.
Saline Preparation: to demonstrate motile trophozoites
Iodine Preparation: to demonstrate killed trophozoites and stained cysts.
- Naked eye examination of stool
Colour: dark brown
Odour: offensive
Bloody mucus: present
Consistency: semifluid
Reaction: acidic
Prevention and Control
- Flies should not be allowed to contaminate food or water.
- Water should be properly boiled before being consumed.
- Raw fruits and vegetables need to be thoroughly cleaned.
- Maintaining personal hygiene is especially important before or during meals.
- The four "F"s—Flies, Food, Finger, and Filth—should be avoided (foul matter).
Treatment
Treatment of Amoebiasis is based on the use of amoebicides.
- Luminal amoebicides (drugs that kill Entamoeba in lumen of intestine)
- Diloxanide furoate
- Di-iodohydroxyquin
- Paromomycin
- Tissue amoebicides (drugs that kill Entamoeba present in liver, intestine or other tissues)
- Emetine
- Dehydroemetine
- Chloroquine (effective only in liver)
- Both luminal and tissue amoebicides
- Metronidazole
- Nitroimidazole
Giardia lamblia
A typical intestinal parasite of humans and other animals, such as dogs, cats, ducks, and so on, is Giardia lamblia. It's a member of the protozoa phylum. It affects predominantly youngsters and is located in the human small intestine (duodenum and upper portion of jejunum). G. lamblia is one of the first infections to infect babies in impoverished nations.
Morphology
G. lamblia exists in two forms which is shown in figure:
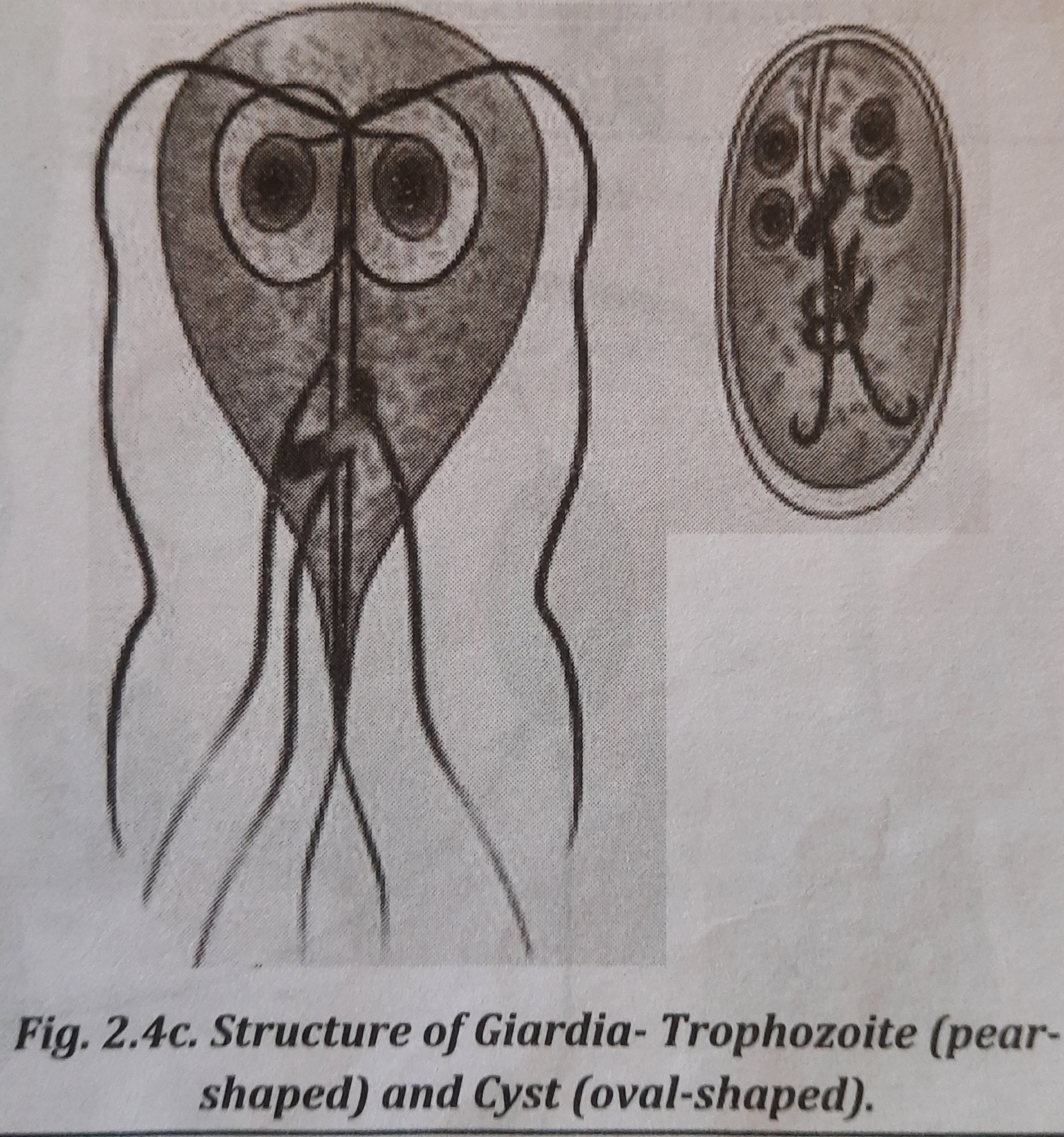
- Trophozoite: It is pear-shaped, measuring 10-20 µm in length and 5-15 um in width. Trophozoite is the diagnostic stage.
- Cyst: It is oval-shaped, measuring 10-14 um x 7-10 µm in size. It is diagnostic as well as infective stage.
Lifecycle
Giardia is a parasite that has only one host, a man, and completes its life cycle there (or other vertebrates).
A man becomes infected when he consumes Giardia cysts (via tainted food, drink, hands, utensils, etc.). The cyst's wall dissolves in the duodenum, where it develops into a trophozoite that divides into binucleated trophozoites by binary fission.
When these trophozoites move down to the colon, they lose their flagella, which leads to encystment. Tetranucleated cysts, which are infectious and expel with feces, are generated during a second phase of division. Giardia's life cycle is seen in the image.
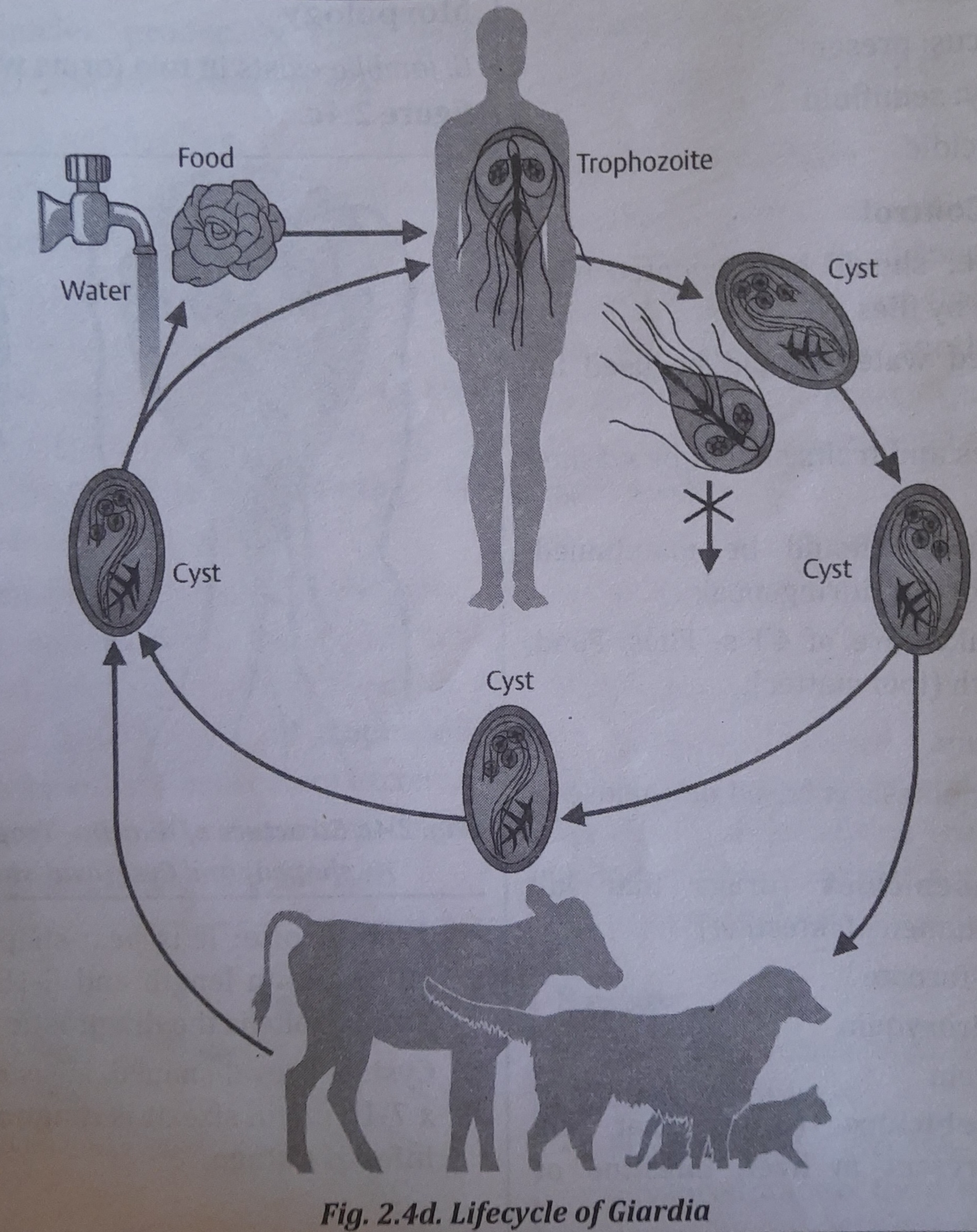
Infective Stage
Tetranucleated cysts are the infective stage which are discharged in faeces of man.
Mode of Infection
When food and water contaminated with cyst is ingested, the host get infection. [Feco-oral route].
Pathogenesis
Infection caused by G. lamblia (G. intestinalis) is known as giardiasis.
Symptoms
Duodenal and jejunal irritation, epigastric pain, flatulence and chronic diarrhea (steatorrhea type), stool is foul smelling, containing mucus and fat but not blood.
Collection of Sample, Processing and Identification
Sample or Specimen: stool and duodenal aspirate.
Microscopy
- Microscopical examination shows cysts in normal stool, and the cysts and trophozoites in diarrhoeal stools.
- Giardia trophozoites may be seen in duodenal or jejunal aspirates.
ELISA and immunochromatographic strip tests: can detect Giardia antigens in faeces.
Prevention and Control
One should take care of 4'F's- Flies, Food, Finger and Filth (foul matter).
- preventing flies from contaminating food and drink.
- As soon as giardiasis is identified, the patient is treated.
Treatment
The drug used for the treatment of Giardiasis are-
- Metronidazole: 250 mg, t.i.d. for 5 days.
- Tinidazole: 2 gm, once. Furazolidone: 100 mg, q.i.d. for 7-10 days.
Cryptosporidium
Cryptosporidium hominis (formerly Cryptosporidium parvum) is intestinal a protozoa which causes cryptosporidiosis. The main symptom of the disease is diarrhea which is most patients. severe in immunocompromised
Morphology
The parasite released in the faeces is known as oocyst. It is spherical or oval, measuring 4-5 μm in diameter.
Life Cycle
- In one host, C. hominis reproduces both sexually and asexually (man, cattle or dog, etc.).
- Man contracts an illness by consuming food and drink that have been tainted with Cryptosporidium oocyst-containing feces.
- The excystment of the oocysts in the intestinal lumen results in the release of sporozites, which infiltrate the epithelial cells and transform into trophozoites.
- Merozoites are created when trophozoites reproduce asexually. Microgametocytes and macrogametocytes, which are a sexual stage, are created when merozoites infect other epithelial cells. Twelve to sixteen microgametes are produced by a macrogametocyte. One macrogamete is transformed by a macrogametocyte.
- A zygote grows into an oocyst after fertilization, which is discharged in feces and spreads the infection from one person to another. It shows the Cryptosporidium life cycle in picture 2.4e.
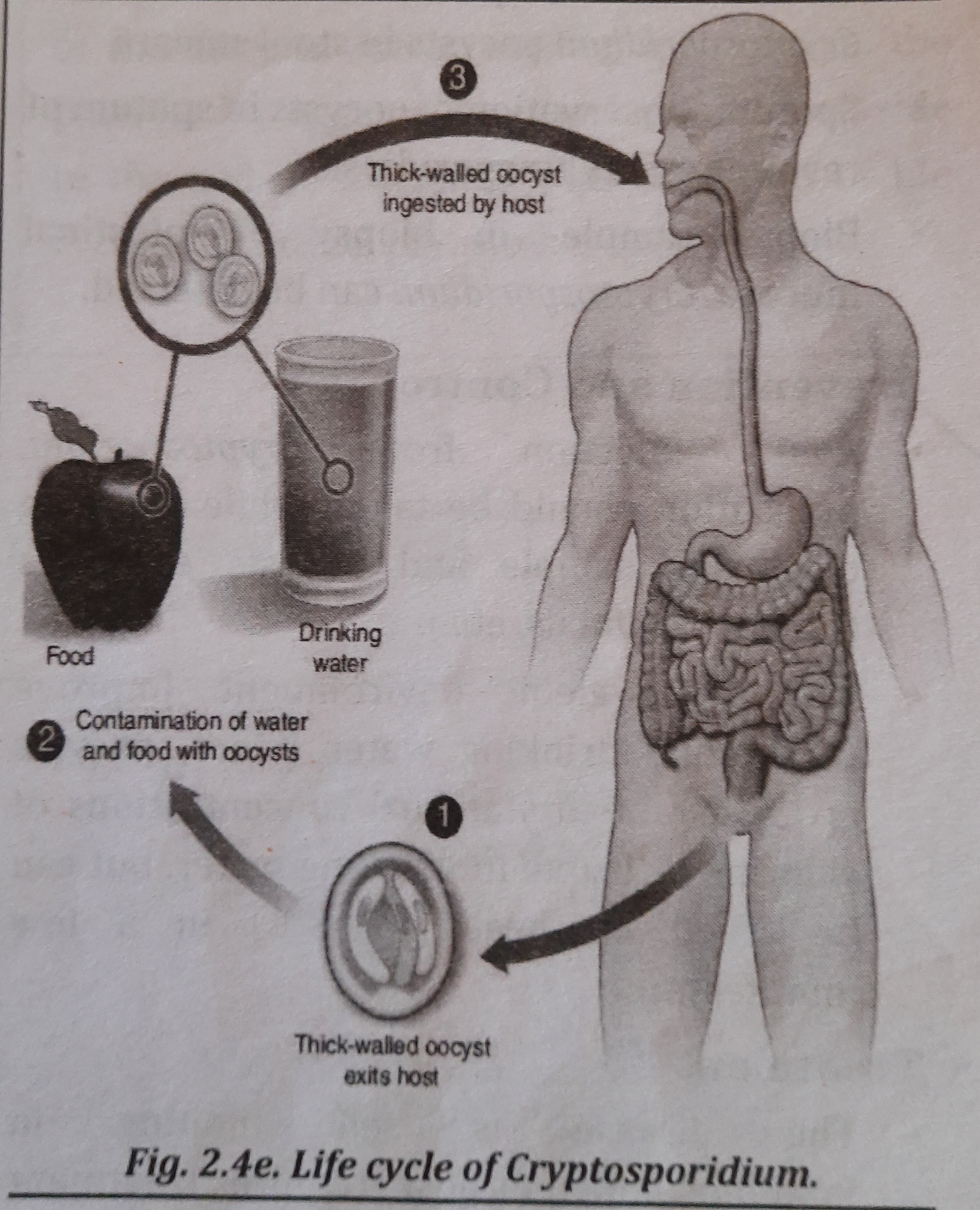
Infective Stage:
Sporulated Oocysts (thick-walled oocyst) are the infective stage.
Mode of Infection
The parasite infect the new host- when infective stage is ingested trough contaminated food and water. by direct contact (sexual practices involving oral-anal contact).
Pathogenesis
Infection caused by Cryptosporidium hominis is known as cryptosporidiosis.
Symptoms
The main symptom of the disease is diarrhea which is most severe in immunocompromised patients (e.g. those with AIDS).
Collection of Sample, Processing and Identification
Sample or Specimen: stool, sputum and sample from intestinal biopsy.
- Stool: Microscopic observation of Cryptosporidium oocysts in stool smears.
- Sputum: Observation of oocysts in sputum of respiratory cryptosporidiosis.
- Biopsy sample in biopsy of intestinal mucosa, Cryptosporidium can be detected.
Prevention and Control
- Avoid being infected with Cryptosporidia by taking precautions when handling diagnostic samples and oocyst excreters (humans, animals, etc.).
- Keep the environment clean. Improve the community's access to clean drinking water since oocysts can survive in water with typical levels of chlorine or ozone but can be quickly destroyed by heat (over 70.8°C).
Treatment
- In an immunocompetent host, the illness is self-limiting (requires treatment only to prevent dehydration).
- However, it is necessary to take 500 mg of nitazoxanide every day for 3-5 days in immunocompromised hosts (but show a poor prognosis).
- Treatment options include antimicrobial medications including azithromycin, spiramycin, and difluoromethylornithine.
Intestinal Worms
- Round worm (Ascaris lumbricoides).
- Hook worm (Ancylostoma duodenale)
- Pin worm (Enterobius vermicularis)
- Whip worm (Trichuris trichura)
- Tapeworm (Taenia solium and Taenia saginata)
- Dog tapeworm (Echinococcus granulosus)
- Dwarf tapeworm (Hymenolepsis nana)
Roundworm
Ascaris is the most common roundworm. It is the endoparasite found in the lumen of small intestine of infected persons.
Morphology:
They are large cylindrical worms, having more pointed anterior than the posterior end. They are pale pink or flesh coloured when freshly passed in stools. Male has its posterior end curved. Female has vulva that leads to a single vagina. Single worm lays up to 200,000 eggs per day. The eggs are passed in faeces.
Size:
Male: 15 to 30 cm in length and 2 to 4 mm in thickness.
Female: 20 to 40 cm in length and 3 to 6 mm thickness.
Morphology of male (6) and female (9) Ascaris is shown in figure 2.5a.
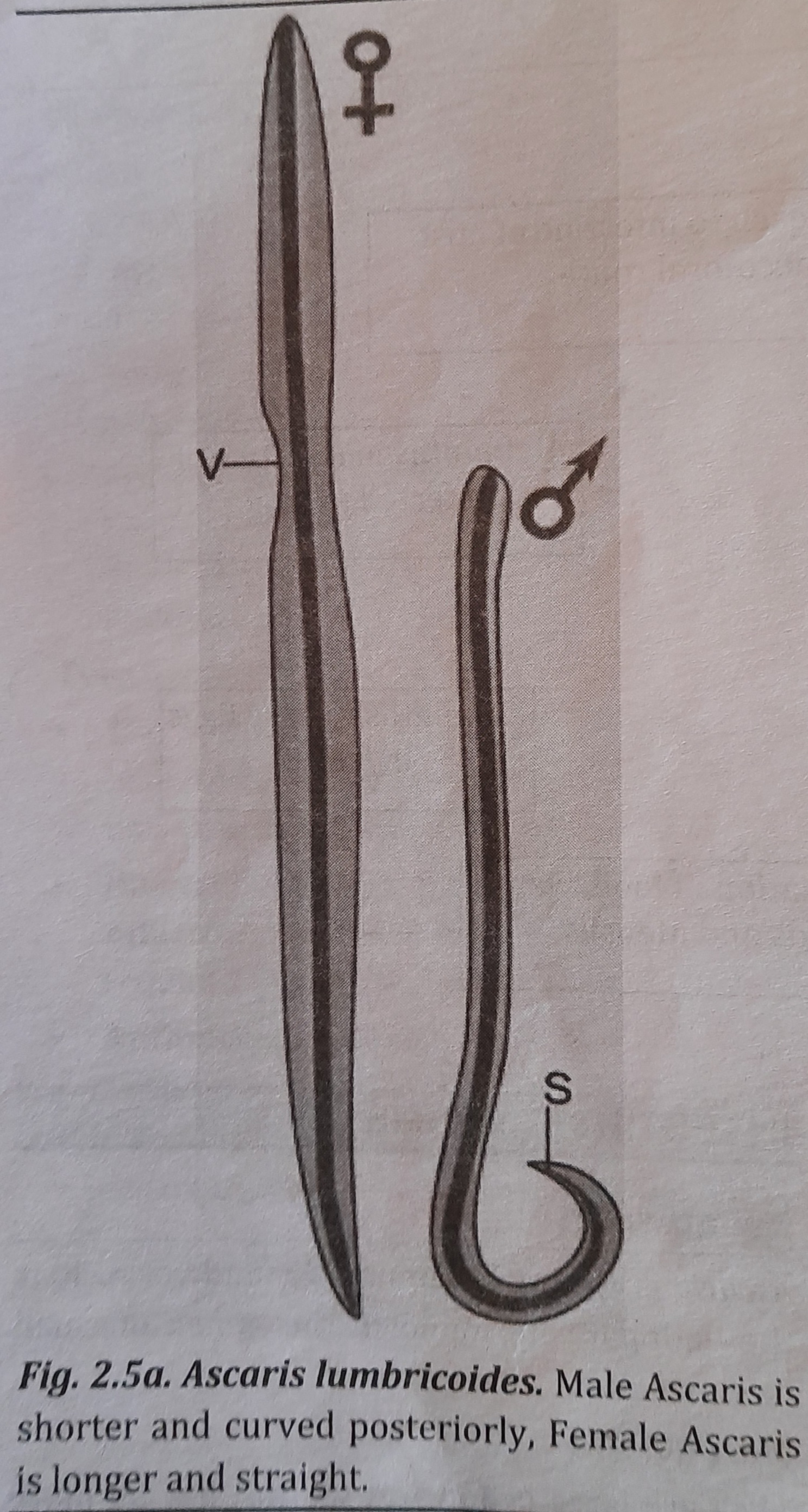
Diagnostic Stage: Eggs in the faeces.
Infective stage: Embryonated eggs (or the second stage larvae).
Life Cycle:
Ascaris is the monogenetic parasite, man is the only single host. Life cycle of Ascaris is explained below:
- In the human small intestine, where female Ascaris deposits hundreds of eggs that are transported by feces, male and female Ascaris reproduce sexually. Because of the body's warmth and low moisture content, Ascaris eggs cannot mature in the gut.
- Finally, in the soil, eggs develop into second stage larvae or embryonated eggs, which are the infectious stage.
- By consuming contaminated food or water that contains embryonated eggs, a new host becomes infected. Larvae develop from eggs that enter the gut.
- The larvae go through the hepatic portal vein to the liver before moving on to the heart and lungs.
- Moulting takes place in the alveoli of the lungs. The larvae then climb to the throat and are ingested to return to the gut. (Achieving sexual maturity is the primary importance of extra intestinal migration.)
- In the human duodenum, the last moulting (development) that results in adult Ascaris takes place.
- Ascaris adult females lay the eggs and complete the cycle. The Ascaris life cycle is seen in figure 2.5b.
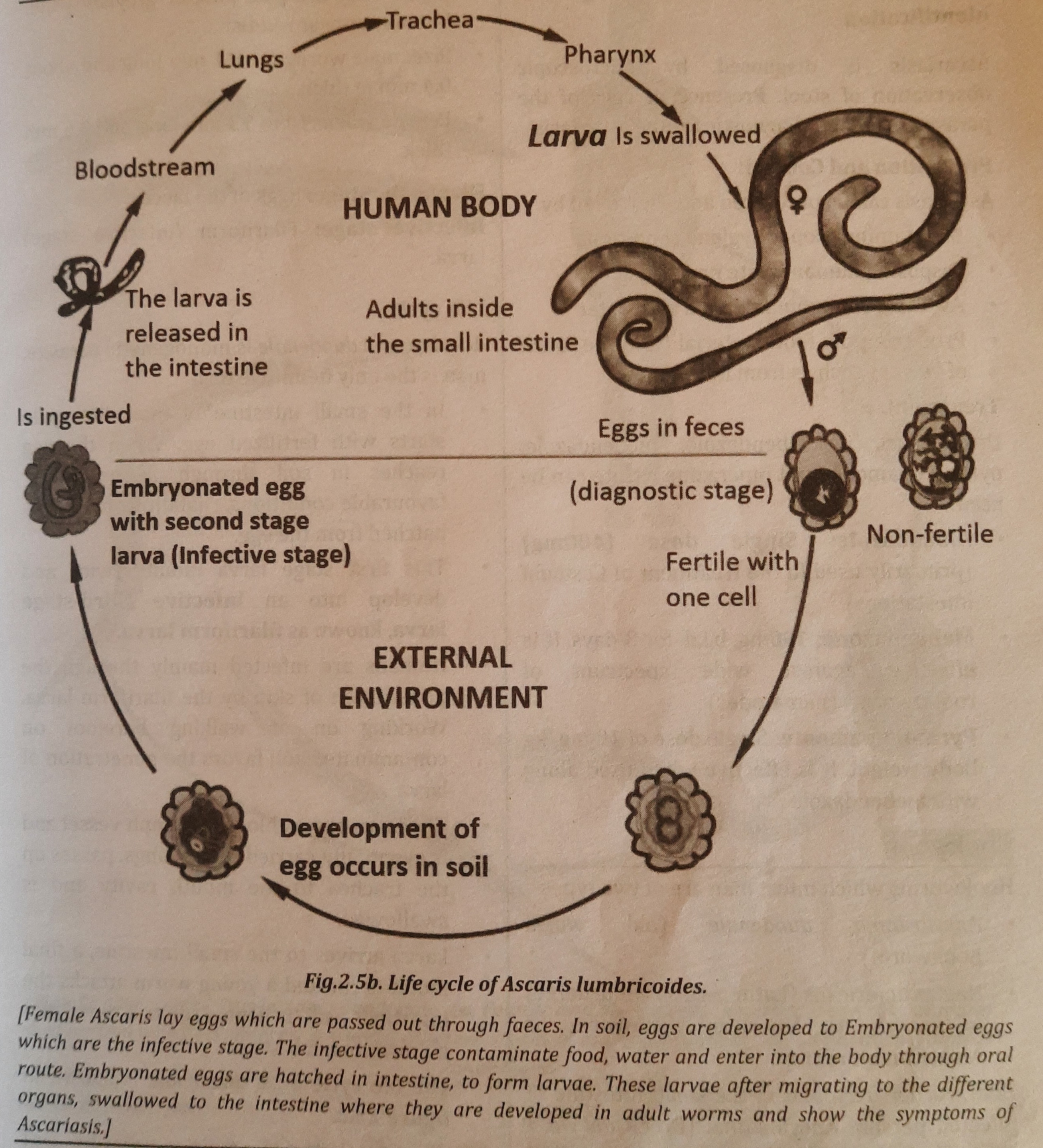
Mode of Infection:
The infective stage of the Ascaris is embryonated egg or rhaditiform larva. Infective stage of the parasite may be ingested with contaminated food or drink through oral (faeco-oral) route.
Pathogenesis:
Adult Ascaris cause ascariasis in man.
Symptoms:
Symptoms of ascariasis include abdominal discomfort, abdominal pain, diarrhoea, vomiting, anemia etc.
Collection of sample, Processing and Identification:
Ascariasis is diagnosed by microscopic observation of stool. Presence of eggs of the parasite in stool is diagnostic for the ascariasis.
Prevention and Control:
- Ascariasis can be prevented and controlled by
- Maintaining proper hygiene conditions
- Disposing human waste properly
- Avoiding contaminated food and water
- Protecting the food material from the touch of vectors such as from houseflies.
Treatment:
Drugs such as albendazole, mebendazole, pyrantel pamoate and piperazine citrate can be used:
- Albendazole: Single dose (400mg) (primarily used in the treatment of Cestodal infestations)
- Mebendazone: 100mg, b.i.d. for 3 days. It is effective against wide spectrum of roundworms (nematodes)
- Pyrantel pamoate: Single dose of 10 mg/kg body weight. It is effective when used along with mebendazole
Hookworm
Hookworms which infect man are of two types:
- Ancylostoma duodenale (old world hookworm)
- Nactar americans (Latin necator= murderer) is known as American hookworm or American murderer.
Hookworms are found in the small intestine of infected person. Ancylostoma (Greek ankylos= hooked; stoma= mouth) has its anterior end which is bent dorsally looking like a hook.
Morphology of Ancylostoma duodenale
- The adult worms live in the small intestines of infected persons, mostly in the jejunum
-
Color: They are pale pink or greyish white, but may appear reddish
- Size: male worm: 8 to11 mm long and about 0.4 mm in thick.
- Female worm:10 to 13 mm long and 0.6 mm thick. Diagnostic stage: Eggs in the faeces. Infective stage: Filariform (infective stage) larva.
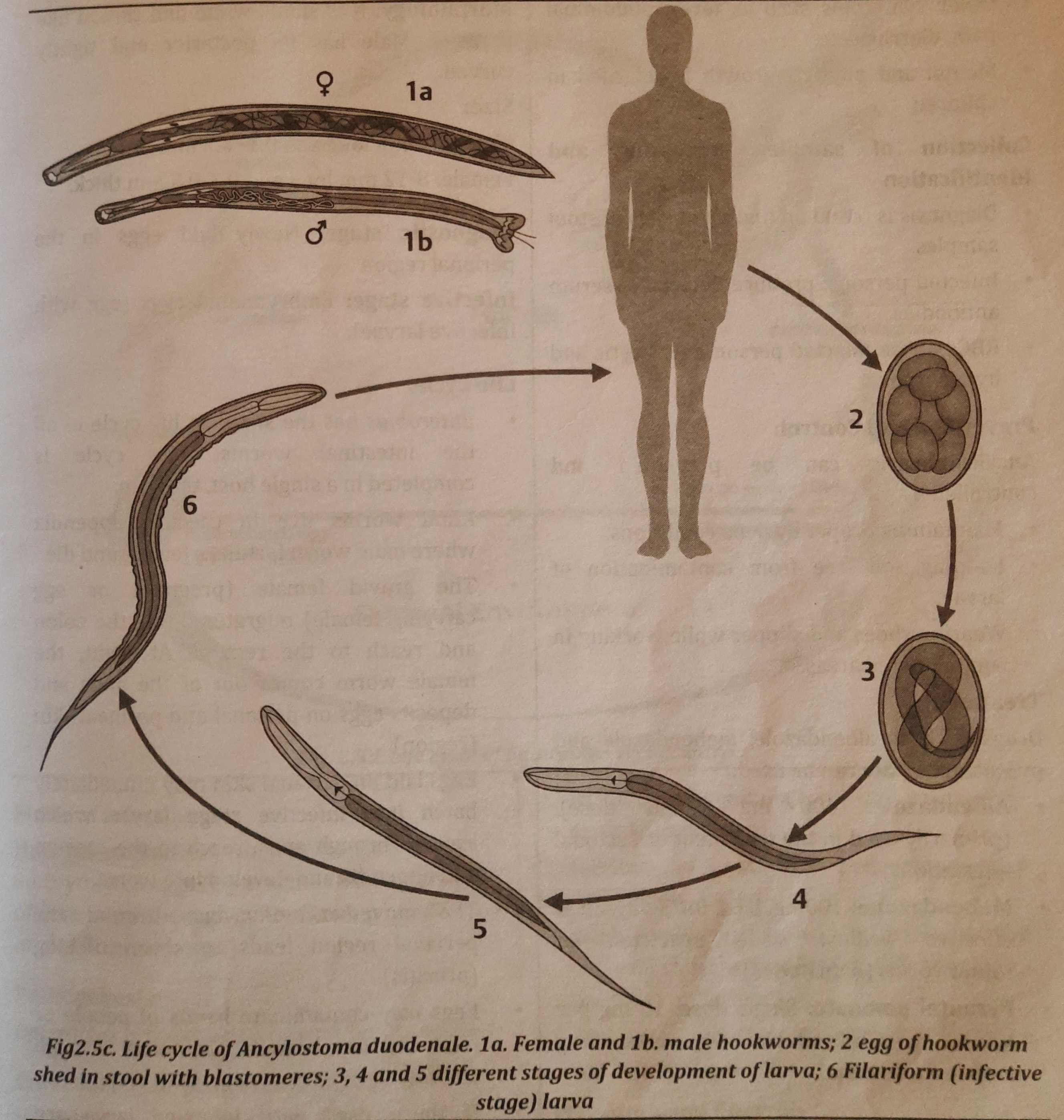
Life cycle
- Man is the sole undisputed host of the monogenetic parasite Ancylostoma duodenale.
- The life cycle of a man's small intestine begins with a fertilized egg. Under ideal circumstances, the egg hatches into a rhabditiform larva when it penetrates the soil through feces.
- This first-stage larva goes through two moults before evolving into the filariform larva, an infectious third-stage larva.
- The primary route of infection for humans is skin penetration by filariform larvae. Working in or barefooting in polluted soil encourages larval penetration.
- The larva enters a blood or lymph vessel, travels via the trachea to the oral cavity, and is then ingested after being delivered to the lungs.
- Arrival of the larva in the small intestine is followed by a final moult and an assault on the intestinal wall by a juvenile worm. It reaches the adult stage and starts sucking blood.
- Figure 2.5c depicts the A. duodenale life cycle.
- About five weeks pass between the introduction of parasites and the first appearance of eggs in the feces.
- The parasites can last between one and fifteen years in the human stomach.
.
Mode of Infection:
The infective stage of the Ancylostoma (hookworm) is Filariform larva. Filariform Larvae may penetrate the skin and enter into blood or lymph.
Even, the infective larvae may enter the body by oral route through contaminated food, fruits and vegetables.
Pathogenesis
Ancylostoma is the causative agent for the disease Ancylostomiasis or hookworm disease in man.
Symptoms
- The effects of larval skin penetration include erythema, burning, and itching.
- a lack of iron anemia
- other signs include a fever, discomfort in the abdomen, diarrhea, etc.
- In youngsters, both physical and mental development is slowed.
Collection of sample, Processing and Identification
- Diagnosis is based on finding of eggs in stool antibodies. samples.
- Infected persons: produce detectable serum hypochromic.
- RBCs of the infected person: microcytic and
Prevention and Control:
Ancylostomiasis can be prevented and controlled by
- preserving sanitary conditions.
- preventing larval contamination of the soil.
- working in contaminated areas while wearing slippers and shoes.
Treatment:
Drugs such as albendazole, mebendazole and pyrantel pamoate can be used:
- Albendazole: 400 mg (Single dose). (primarily used in the treatment of Cestodal infestations)
- Mebendazone: 100mg, b.i.d. for 3 days. It is effective against wide spectrum of roundworms (nematodes)
- Pyrantel pamoate: Single dose, 10 mg per kg for 3 days. It is effective when used along with mebendazole.
Pinworm
The scientific name of the pinworm (threadworm) of man is Enterobius vermicularis which is present in the caecum, appendix and adjacent portion of ascending colon.
Morphology:
It is small, white and thread like parasite. Male has its posterior end tightly curved.
Size:
Male: 2-4 mm long and 0.1-0.2 mm thick. Female: 8-12 mm long and 0.3-0.5 mm thick.
Diagnostic Stage: Newly laid eggs in the perianal region
Infective Stage: Embryonated eggs (egg with infective larvae).
Life Cycle:
- The life cycle of Enterobius is the simplest of all the intestinal worms. The entire life cycle takes place in one host, the human.
- The caecum, an appendix, is where adult worms dwell and die after fertilizing the female.
- The gravid female migrates through the colon and reaches the rectum when she is gravid (carrying eggs or pregnant). The female worm emerges from the anus at night and lays eggs on the skin of the perianal and perineal regions (region).
- Infectious stage larvae that are placed on the perianal skin may instantly hatch into worm-like organisms after ascending via the anus and reaching the caecum and appendix.
- Severe itching is brought on by the worm's movement in the perianal area (pruritis).
- Eggs have the potential to infect hands, food, and the air through clothing.
- If these eggs containing infectious larva are ingested, the embryonated eggs in the gut hatch into larvae that go to the caecum and appendix where they mature into adult worms. Figure 2.5d shows an illustration of the Enterobius life cycle.
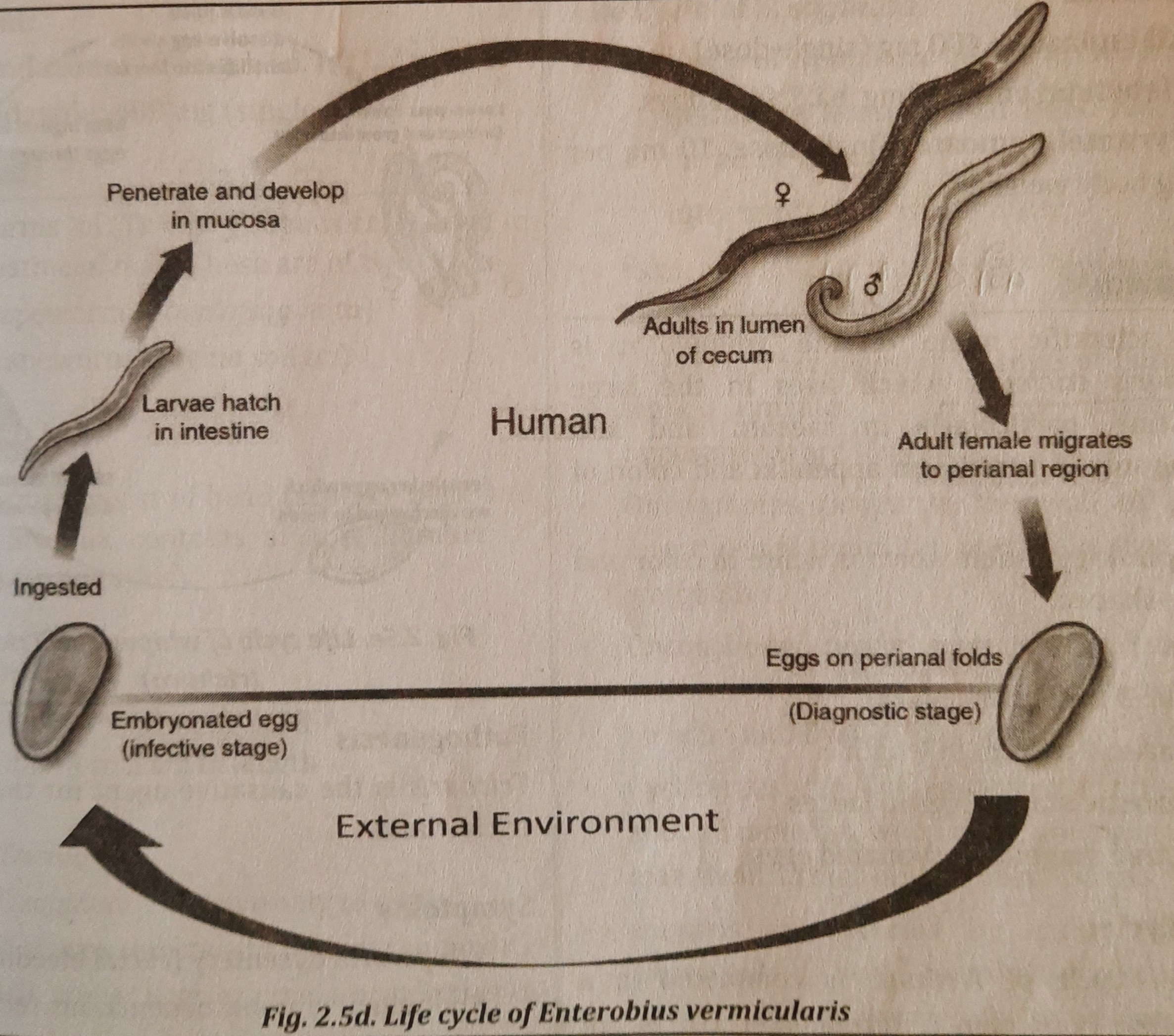
Mode of infection:
Humans are infected when they ingest embryonated eggs (eggs with infective larvae) of Enterobius through contaminated hands, food or clothing (air borne).
Pathogenesis
Enterobius is the causative agent for the disease
Enterobiasis or pinworm infection.
In girls, female worms may enter into vagina and urethra leading to vaginitis and urethritis.
Symptoms
- severe perianal itch, irritation, and restlessness. abdominal discomfort, diarrhea, lack of appetite, bedwetting, and teeth grinding.
- Pinworms can enter female genitalia and irritate and inflame them.
- Early sexual desire in girls may result from vaginal irritation.
Collection of sample, Processing and Identification
Enterobiasis can be prevented and controlled by
- swabs collected from the perianal and perineal skin (early morning, before going to the toilet or bathing).
- The eggs or adult worms may sometimes be noticed on the surface of stools.
Prevention and Control:
Enterobiasis can be prevented and controlled by
- keeping up good hygienic practices (helps in controlling reinfection)
- washing and cleaning nightwear and underwear.
- frequent bathing and maintaining a clean genital area.
Treatment:
- Pyrantel pamoate: Single dose, 10 mg per kg body weight.
- Mebendazone: 100mg, b.i.d. for 3 days.
- Albendazole: 400 mg (single dose).
Tapeworm
Adult worms of Taenia (Tapeworms) live in small intestine of man. These are of two types-
- Beef tapeworm (Taenia saginata)
- Pork tapeworm (Taenia solium)
Morphology
Adult worms consist of head (scolex), neck and strobila. Strobila contains a large number of segments (proglottids).
Size of Taenia
- T. saginata: 3 to 4 cm in length
- T. solium: 4 to 5 cm in length
Eggs of T. saginata: infective only to cattle.
Eggs of T. solium: infective to both pig and man.
Diagnostic stage: Eggs or gravid proglottids in faeces.
Infective stage:
Cysticercus (Encysted larval stage) in muscle.
Infective stage for
- T. saginata is- Cysticercus bovis
- T. solium is- Cysticercus cellulosae
Life Cycle of T. saginata
- Life cycle of T. saginata occurs in two host.
- definitive host: is man (who harbours the adult worm)
- intermediate host: is cattle.
- Feces are dispersed together with eggs or the gravid Taenia segments.
- Cows or buffaloes consume these eggs while grazing. The oncospheres are released in the duodenum.
- Oncospheres pass through the small intestine's wall and enter the portal or lymphatic circulation.
- Oncospheres are filtered out in muscle where they grow into Cysticercus bovis before reaching the general circulation.
- When consumed by humans, cysticercus can survive for up to 8 months in the meat of cattle before continuing to grow.
- By consuming beef that has cysticercus bovis uncooked or undercooked, humans can contract the disease. In tiny intestine-forming larvae, hatching takes place.
- In two to three months, the larvae reach sexual maturity and begin to lay eggs. The cycle is maintained by the passing out of eggs and gravid proglottids in feces. Figure 2.5f depicts the Taenia saginata life cycle.
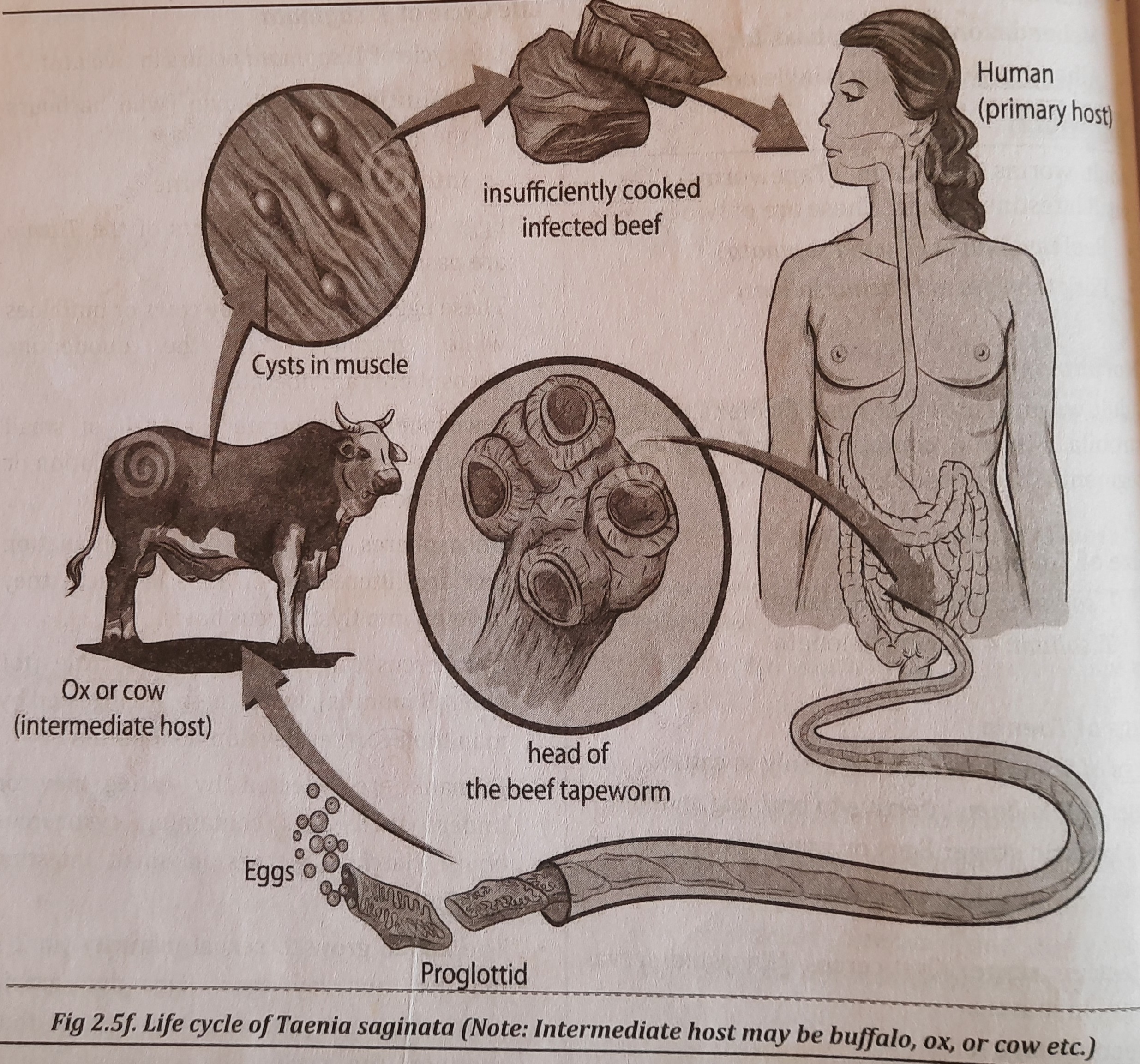
Life Cycle of T. solium
- It is a largest endoparasite found in the small intestine of man. T. solium is also a digenetic parasite.
- definitive host: is man (who harbours the adult worm).
- intermediate host: is pig.
- The small intestine of a man is home to adult worms, which release their eggs in the feces. Pigs consume eggs or gravid segments (coprophagus animal). The oncospheres are released in the duodenum.
- Oncospheres enter the systemic circulation after penetrating the small intestine's wall and stay in striated muscles.
- They mature into the encysted larval stage in muscle (Cysticercus cellulosae).
- By consuming contaminated pork that is raw or undercooked, a man might get Cysticercus cellulosae.
- These larvae develop in the small intestine, reach sexual maturity, and then lay eggs in two to three months. Faeces include eggs and gravid proglottids, continuing the cycle seen in figure 2.5g.
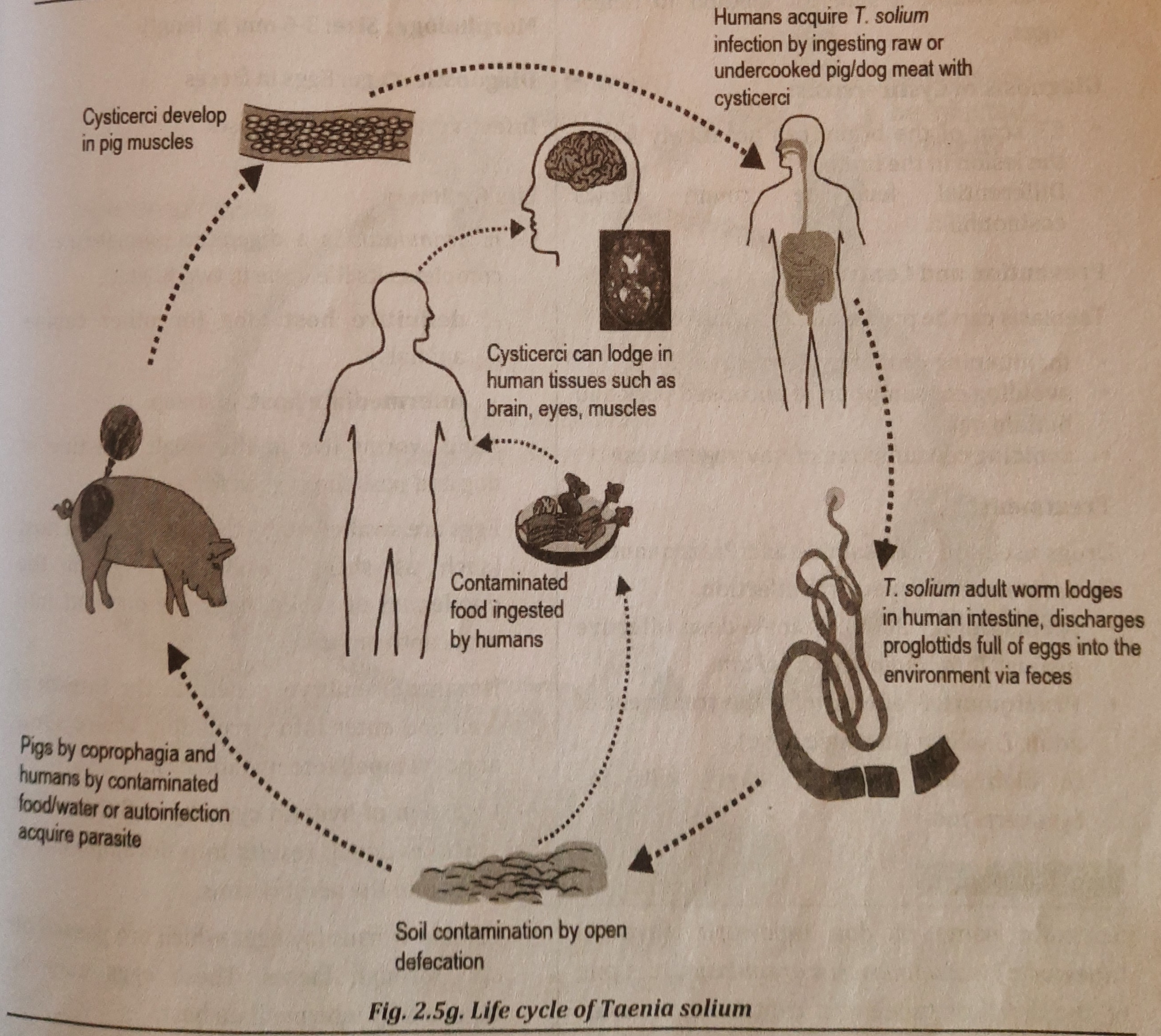
Mode of Infection of Taenia
Man acquire infection by eating raw or undercooked meat containing cysticercus larvae
Pathogenesis (T. saginata and T. solium)
- Adult tapeworms cause taeniasis.
- Larvae of Taenia solium cause cysticercosis
Symptoms
- Abdominal discomfort, abdominal pain,. bowl irregularity, nausea, headache etc.
-
Cysticercus larva causes cysticercosis. If the larvae settle in eye, they may cause blindness. In brain, they cause necrosis of the brain tissue resulting into epileptic attack (epilepsy).
Collection of sample, Processing andIdentification
Diagnosis of Taeniasis:
Collection of sample:
stool and anal swab.
- Stool examination: to detect presence of eggs in stool
-
Anal swabs: a superior method to detect eggs.
Diagnosis of Cysticercosis:
- CT scan of the brain: can accurately locate the lesion in the brain.
- Differential leucocyte count: shows eosinophilia.
Prevention and Control
Taeniasis can be prevented and controlled by:
- preserving a clean environment.
- avoiding eating undercooked hog and buffalo meat as well as uncooked veggies.
Treatment
Drugs used are Nicolsamide and Prazoquantel - for treatment of Tapeworm infection.
- Nicolsamide- 2000mg single dose, effective against T. saginata and T. solium.
- Praziquantel- effective for the treatment of adult T. solium (in single dose).
In high dose (for 3-7 days), kills the cysticerci too.
Things to remember
© 2021 Saralmind. All Rights Reserved.

 Login with google
Login with google