Heat
Subject: Science

Overview
Heat refers to the amount of heat energy transferred between objects, causing a drop in one's thermal energy and an increase in another. Heat transfers occur through conduction, convection, and radiation processes. Heat causes atoms and molecules to vibrate more vigorously, leading to increased volume and temperature. Water has a unique anomalous expansion, with its density falling and volume rising when heated or cooled to 40°C. The temperature of water decreases with temperature, with the highest density at 40°C. The specific heat capacity of a substance is the amount of heat needed to raise its temperature by 1°C for a mass of 1 kg. Different materials have different specific heat capacities, with water having the highest specific heat capacity. Water can effectively cool heated items, such as automobile radiator coolants and thermal power plants.
The molecules have high kinetic energy because of their fast motion. When steam comes into contact with human skin, its molecules can readily pass through the skin and burn the internal tissues. This hurts a great deal.
A thick glass tumbler will fracture when hot water is abruptly poured into it because the heat from the interior cannot be transmitted to the exterior, and the increased volume inside the glass puts pressure on the exterior. The water volume grows and the glass bottle fractures when a glass bottle filled with water is placed in the deep freeze. In a similar vein, the pace at which temperature increases varies among heated items. When instant boiling water is added to the steel glass we are holding, the glass's temperature rises and our hands get burned.
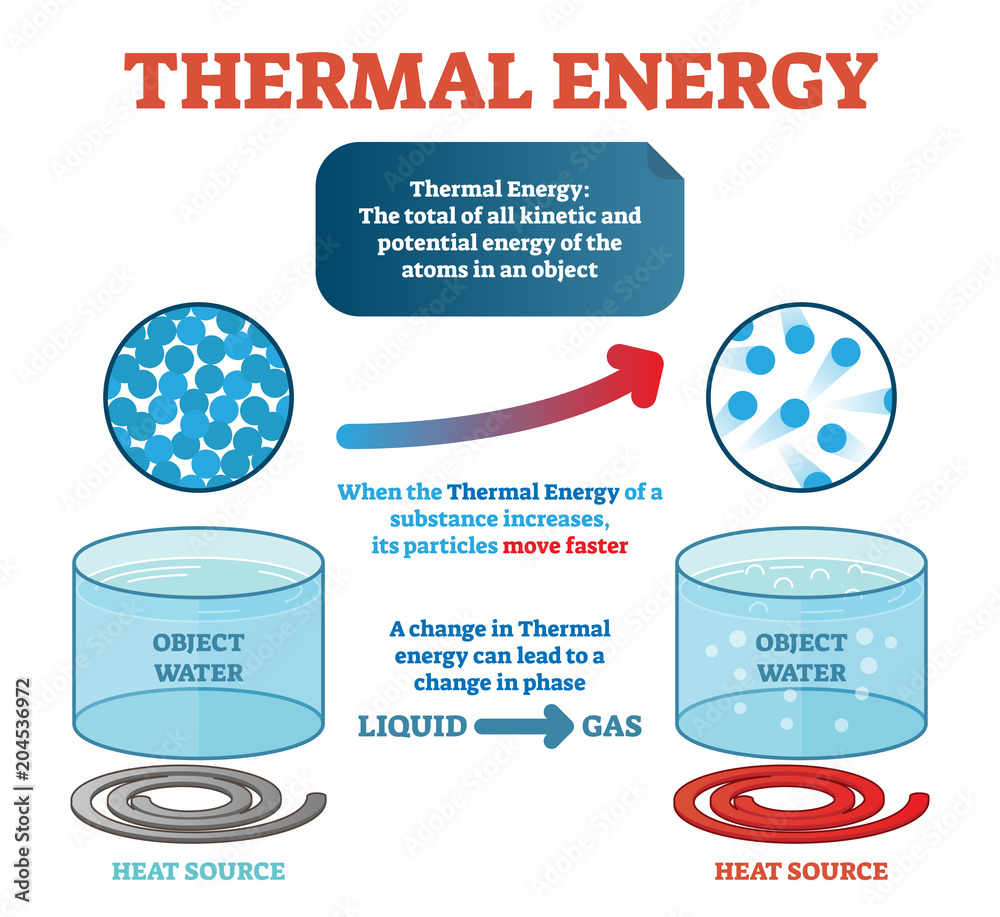
Thermal Energy
Numerous atoms or molecules, make up matter. These molecules or atoms, are in constant motion. In comparison to molecules in cold water, molecules in lukewarm water have a higher kinetic energy. Molecules in hot water move even more quickly than they do in lukewarm water. The thermal energy is the sum of the molecules' kinetic energies. The thermal energy of hot water is maximum and that of cold water is lowest when the masses of warm water, tap water, and cold water are equal.
The molecules of boiling water move more quickly due to the external energy. A substance's atoms and molecules all have different kinetic energies at different times. Their average kinetic energy is, therefore, significant. The average kinetic energy of a substance's atoms or molecules is what determines its temperature. A substance's temperature reveals the average kinetic energy of its molecules. Although degrees Celsius (°C) are the most widely used unit of measurement for heat, kelvin (K) is the SI unit. It is determined by means of a thermometer.
Since the temperature of the lukewarm water in the bucket is 30°C and the boiling water in the pan is 100°C, the boiling water in the pan has an average kinetic energy that is significantly higher than the lukewarm water in the bucket. However, there are significantly fewer water molecules in the pan than there are in the bucket. Because there are more molecules in the lukewarm water, even if the average kinetic energy of the molecules in the boiling water in the pan is higher, the total kinetic energy of the molecules is higher. As a result, the thermal energy of the lukewarm water in the bucket is greater than that of the boiling water in the pan. A substance's mass and average molecular speed determine its thermal energy.
Thermal energy is transferred from the boiling water to the lukewarm water when the two previously described liquids are combined. Heat, then, is the quantity of thermal energy that is transferred from one location to another as a result of a temperature differential. The joule (J) is the SI unit of heat, just like other forms of energy. A calorimeter can be used to measure it.
Heat
Nothing can hold heat. It simply refers to the amount of heat energy that is moved from one thing to another. One object's thermal energy drops and the other's increases as heat is transferred. As a result, as heat is transferred, one object's temperature drops while another rises.
Three different processes—conduction, convection, and radiation—are used to transfer heat. When we touch anything, it feels hot if heat enters our body from it and cold if heat exits.
When two bodies reach the same temperature, heat moves from one that is hotter to one that is colder.
Effect of Heat on Volume
The following graphs illustrate the relative differences in atom/molecule lengths between solid, liquid, and gaseous substances.
Because of their mutual attraction, solid matter molecules are held together in a certain way. But they are not motionless; rather, they vibrate continuously. Heat causes the atoms and molecules of a solid to vibrate more vigorously. An ongoing source of heat causes the atoms and molecules to vibrate quickly. For this reason, as the volume rises, the force of attraction between them decreases and they move apart. On the other hand, average speed drops as an item releases thermal energy. As a result, the object contracts and the temperature drops. The majority of materials expand with rising temperatures and shrink with falling ones.
Anomalous Expansion of Water
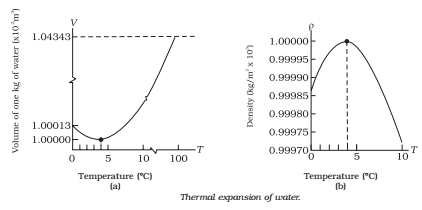
The volume of most substances rises with heat. Water, though, has a distinct character. When heated, its volume rises, but when heated from 0°C to 4°C, it falls. Additionally, when it cools to 4°C, its volume drops; nevertheless, as it rises from 4°C to 0°C, it expands. Consequently, the smallest volume and greatest density of water are found at 4°C.The anomalous expansion of water is the term used to describe this special quality of water.
The relationship between water density and volume is seen in the graph in Figure. The water's density falls and its volume rises when it is heated or cooled to 40 °C.
Effects of Anomalous Expansion of Water
Throughout the winter, the ambient temperature in all frigid climates falls below freezing. The water's temperature drops in tandem with the environment's decline in temperature. Water has the highest density when the temperature hits 40°C. It therefore plummets to the bottom. Beyond this, layers of water form at temperatures of 3°C, 20°C, 1°C, and 0°C, respectively, from the bottom to the top of the ice. The surface water freezes and floats on the top of the water below when it hits 0°C. Fish and other aquatic life can thus live in the water beneath the ice.
In extremely cold climates throughout the winter, when the ambient temperature steadily drops, the amount of water within the pipe rises and presses on the inner wall. The pipe may break due to the extremely high pressure that results from the water freezing and turning into ice. A water bottle kept in the deep cold will fracture for the same reason.
Specific Heat Capacity
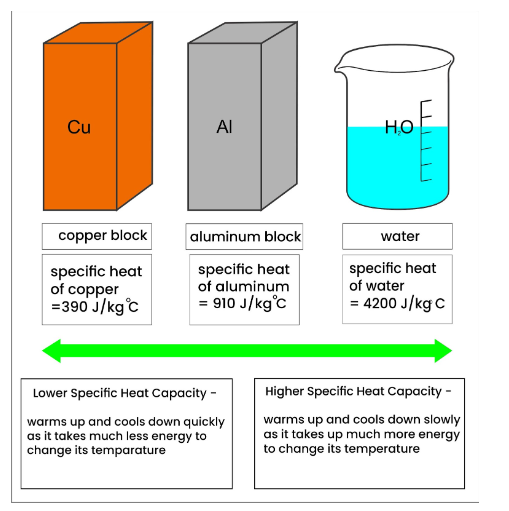
The specific heat capacity of a substance is defined as the amount of heat needed to raise its temperature by 1°C for a mass of 1 kg. The SI unit for it is kg°C, or joule per kilogram.
Factors Influencing a Matter's Ability to Absorb Heat
The condition of the items can affect how quickly and how much heat they absorb, even if they are composed of the same material but have different forms.
The quantity of heat absorbed by a substance (Q) is directly proportional to its mass (m)
Q α m.................................(i) [Keeping the temperature constant]
The quantity of heat absorbed by a substance (Q) is directly proportional to the change or increase in temperature (T2-T1).
Q α (T2 - T1).......................(ii) [Keeping the mass constant]
Combining (i) and (ii)
Q α m (T2-T1)
Q = ms (T2- T1)..................(iii) where s is a constant and refers to the specific heat capacity of the substance.
Therefore, the heat absorbed or released by a substance is equal to the product of the mass (m), the specific heat capacity (s) and the temperature change (T2-T1) of that substance. This equation is called the heat equation.
From equation (iii)
S = \(\frac Qm\) (T2-T1)
If the mass of the substance is m = 1kg and the temperature change due to the quantity of heat absorbed or released is 10°C, then s = Q
Depending on the substance, different materials have different specific heat capacities. This table lists a few materials along with their particular heat capabilities.
| Substances | Specific Heat Capacity | Substances | Specific Heat Capacity |
| Water | 4200 | Aluminum | 884 |
| Ethyl Alcohol | 2400 | Iron | 460 |
| Kerosene Oil | 2010 | Copper | 385 |
| Ice | 2100 | Silver | 236 |
| Mercury | 126 | Gold | 130 |
Based on the specific heat capacity listed in the above table, 4200 J of heat is needed to raise the temperature of one kilogram of water by one degree Celsius. In a similar vein, 2100 watts of heat are needed to raise the temperature of every kilogram of ice by one degree. Here, the specific heat capacities of the ice and the water differ despite being two states of the same substance.
Uses of Specific Heat Capacity
The water has an extremely high specific heat capacity. For every kilogram of water, 4200 J of heat is absorbed or released, resulting in a 1°C temperature shift. Water may effectively cool heated items due to its features. For instance, automobile radiator coolants are made of water. Despite absorbing a lot of heat from the car's engine, the water's temperature does not rise much. For this reason, thermal power plants that generate electricity also require water as a coolant.
Things to remember
- The quantity of heat energy that is transmitted between things, resulting in a decrease in one's thermal energy and a rise in another, is called heat.
- The heat equation is defined as the heat absorbed or released by a substance being equal to the product of the mass (m), the specific heat capacity (s) and the temperature change (T2-T1) of that substance.
- The anomalous expansion of water occurs when the water's density falls and its volume rises when heated or cooled to 40°C.
- Specific heat capacity is defined as the amount of heat needed to raise its temperature by 1°C for a mass of 1 kg.
- the specific heat capacities of the ice and the water differ despite being two states of the same substance.
© 2021 Saralmind. All Rights Reserved.




 Login with google
Login with google